 |
A long-standing glaucoma patient presented in March for her regularly scheduled follow-up visit. We scheduled her for anterior segment OCT, gonioscopy, and ultrasound biomicroscopy (UBM), as well as the standard intraocular pressure (IOP) check and nerve evaluation. She is a 71-year-old Caucasian woman who has seen me for many years. She has glaucoma in her right eye and is a glaucoma suspect for her left eye. Current glaucoma medications include Travatan Z HS (Travoprost, Novartis) OD, for which she reports good compliance. Her only other systemic medication is lisinopril and she reports no known allergies to medications.
History
We initially saw her in 2003, when we diagnosed her as a glaucoma suspect based on asymmetric optic nerve cupping. At that time, her threshold visual fields were normal, with no evidence of early field loss, applanation tensions averaged 19mm Hg OD and OS, and cup-to-disc ratios were estimated to be 0.5 x 0.6 OD and 0.3 x 0.35 OS. Her central corneal thicknesses were 588µm OD and 567µm OS when initially obtained. She was hyperopic and initially presented with opened, but slightly narrowed, angles. Over the next several years, she began to develop age-related nuclear cataracts. With time, her angles narrowed a bit, but always remained open and non-occludable. Post dilation IOPs did not alter from predilation IOPs. She remained stable until 2014, at which time she demonstrated manifest changes to her neuroretinal rim in her right eye and subtle visual field deficits corresponding to the rim changes. That’s when we started her on Travatan Z.
At the 2017 visit, her applanation pressures measured at 14mm Hg OD and 16mm Hg OS. Her visual fields were stable when evaluated five months earlier. Cup-to-disc ratios were also stable when evaluated then and were judged to be 0.6 x 0.65 OD and 0.3 x 0.35 OS. Heidelberg Retina Tomograph (HRT 3) imaging and optical coherence tomography (OCT) imaging of the RNFL and macular ganglion cell layers were stable. Visual acuity was 20/30- OD, 20/30+ OS and 20/25- OU. Her cataracts at this point were estimated to be grade 1 anterior and posterior cortical, as well as grade 1 nuclear in configuration.
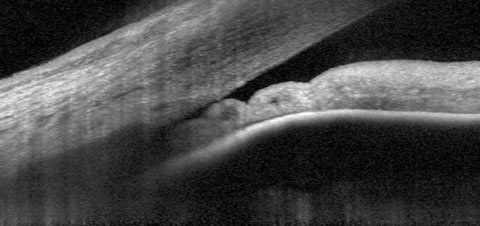 |
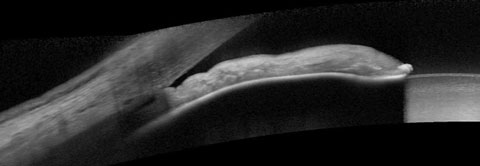
| Fig. 1. At top, our 74-year-old patient’s anterior segment OCT image from 2015 shows narrowing of the temporal angle. Fig. 2. Below, two years later, the narrowing of the temporal angle has progressed. |
 |
A slit lamp examination of her anterior segments was unremarkable except for narrowing of the anterior chamber angles by Von Herick, with the narrowest angles seen nasally and temporally in both eyes, followed by the superior angle, with the widest angles being inferiorly OU. The posterior segment was characterized by bilateral PVDs of several years’ duration, fine RPE granulation in the central maculae and normal retinal vasculature. Previous dilated fundus examinations demonstrated intact peripheral retina.
The focus of this visit was to evaluate her angles, in particular their gradual narrowing over the past few years as her cataracts developed. Anterior segment OCT, UBM and gonioscopy were all performed.
Discussion
Optometrists have a couple options for dealing with patients such as this one. A prophylactic laser peripheral iridotomy (LPI) procedure, for instance, can create a communication channel between the anterior chamber and the posterior chamber, which is extremely important when pupillary block is a possibility. Another option is to monitor these patients closely for signs or symptoms associated with subtle episodes of angle closure, as we’ve done in this instance.
The key to managing these patients is to obtain a good perspective of the angle as seen gonioscopically, and other relevant angle structures, such as the iris and the position of the ciliary body in reference to the iris and the angle. These last two perspectives are best given by anterior segment OCT imaging and UBM imaging.
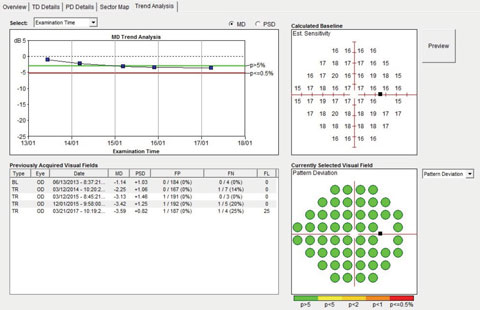 |
| Fig. 3. Although we could see the patient had narrow angles, the visual fields over a four-year period remained stable. |
Reading the Images
In our patient’s anterior segment OCT image, taken in 2015, we were able to see a magnified view of the temporal angle in the right eye (Figure 1). You can easily see the close proximity of the peripheral iris to the trabecular meshwork, as well as some adjacent collecting channels. Not surprisingly, with the structures positioned in this image, gonioscopic examination of the angle would show a narrowed angle, with obscuration of the trabecular meshwork, consistent with the appearance of an anatomically narrowed angle. The angle configuration varies somewhat throughout the 360 degree structure, but at its narrowest, other OCT positions are similar to the image shown.
In a 2017 image of the same sector of angle, the angle structures demonstrate even further narrowing, even to the point of obstruction of the trabecular meshwork (Figure 2). While the patient’s optic nerves and fields have remained stable, the angle is progressively narrowing (Figure 3). But what is not readily visible in these images is the ciliary body. This is where UBM imaging comes into play (Figure 4).
The current UBM finding is consistent with UBMs previously performed on this patient, and her plateau iris configuration has remained unchanged. But the important point here is that, from a slit lamp perspective, from a gonioscopic perspective and from an anterior segment OCT perspective, this patient appears to have a straightforward presentation of anatomically narrow angles, perhaps worsening by the increasing axial length of the cataractous lens.
Interpreting the Data
LPIs have little effect on plateau iris induced narrow angles. However, in many patients with plateau iris configurations, not all quadrants demonstrate a plateau iris configuration, as is the case for this patient. Narrow angles are complex, dynamic structures that can change over time. In most cases, angle closure is due to pupillary block and is amenable to peripheral iridotomy. But, optometrists are tasked with considering all potential causes of narrow angles, including plateau iris, lens vault and increased thickness of the lens with cataract progression, scleral buckles, malignant glaucoma and even changing iris thickness.1-3
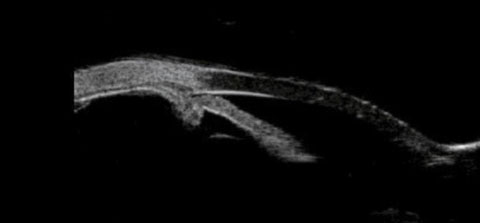 |
| Fig. 4. This UBM scan shows the patient’s plateau iris configuration and anterior positioning of the ciliary body. |
To find the applicable cause or causes, obtain all the imaging you can. Gonioscopy, anterior segment OCT and UBM technology are all in the mix. The classification that we use to describe narrow angles is not uniform, and often there are multiple mechanisms in place with each individual.4,5 Any angle that is not closed is by definition open, and they can have many faces and many degrees of openness. But an angle that is narrow, while technically still open, may eventually close. Gathering as much information as possible to fully assess the narrow angle is the best way to ascertain the risk of closure.
The patient in this case—though her cataracts were not visually debilitating—was best served now with cataract extraction and IOL implantation, especially in light of research that shows cataract extraction’s effect on lowering IOP are greatest in patients with angle closure.6
|
1. Quigley HA, Silver DM, Friedman DS, et al. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma. 2009;18(3):173-9. 2. Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol. 2011;129(10):1283-90. 3. Aptel F, Denis P. Optical coherence tomography quantitative analysis of iris volume changes after pharmacologic mydriasis. Ophthalmology. 2010;117(1)3-10. 4. Niwas S, Lin W, Bai X, et al. Automated anterior segment OCT image analysis for angle closure glaucoma mechanism classification. Comput Methods Programs Biomed. 2016 Jul;130:65-75. 5. Sun X, Dai Y, Chen Y, et al. Primary angle closure glaucoma: what we know and what we don’t know. Prog Retin Eye Res. 2017 Mar;57:26-45. 6. Thomas R, Walland M, Thomas A, Mengersen K. Lowering of intraocular pressure after phacoemulsification in primary open-angle and angle-closure glaucoma: A bayesian analysis. Asia Pac J Ophthalmol. 2016 Jan-Feb;5(1):79-84. |

