 |
In late August, a 79-year-old Caucasian male presented to the office looking for a second opinion regarding his glaucoma. He was told that he should get “laser surgery,” but did not want to proceed with it until obtaining a second opinion from me. His history was significant with an approximate six-year history of glaucoma. He was medicated in both eyes with Travatan Z (travoprost ophthalmic solution, Novartis) HS and Simbrinza (brinzolamide/brimonidine tartrate ophthalmic suspension, Novartis) BID. Other systemic medications included only an unknown inhaler for chronic obstructive pulmonary disease.
He had been seeing the other provider for several years and underwent several medication changes until settling on his regimen. It was unclear exactly why further intervention was recommended; it may have been progressing structural changes to his optic nerve, progressive visual field loss, fluctuation IOP or any combination.
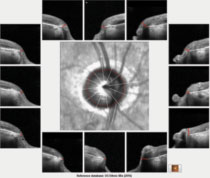 |
| Fig. 1. The 12 o’clock hour BMO scans of the patient’s right optic nerve, all of which are aberrant as compared with a reference database. |
Diagnostic Data
His best-corrected visual acuity was 20/40-OD, OS, OU through minimally hyperopic astigmatic correction. Pupils were reactive to light, and there was mild physiological anisocoria present, along with pupillary ruff disruption in his left eye, most likely as a minor complication from cataract surgery. Extraocular muscles were full in all positions. Applanation tensions were 10mm Hg OD and 14mm Hg OS. Pachymetry readings were 594µm OD and 591µm OS.
The anterior segment was essentially unremarkable, with wide open angles, clear corneas, save for mild arcus OU. Posterior chamber IOLs were present in both eyes centered in the capsular bags, with clear and intact posterior capsules.
Through dilated pupils, bilateral posterior vitreous separations were visible. Stereoscopic examination of his optic nerves revealed an estimated cup-to-disc ratio of 0.90 x 0.90 OD and 0.80 x 0.90 OS. The neuroretinal rims were thin and consistent with advanced glaucomatous damage. The retinal vasculature was characterized by mild arteriolarsclerotic retinopathy in both eyes. Both maculae were characterized by having retinal pigment epithelium granulation and drusen, and mild epiretinal membranes. These macular findings were consistent with the acuities of 20/40 noted earlier. His peripheral retinal evaluations were normal.
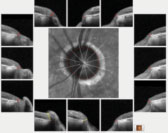 |
| Fig. 2. The left optic nerve BMO overview scan, with most sectors well outside reference database norms. |
A Closer Look
Since he was after a second opinion, we needed more data about the first; in particular the rationale for recommending laser surgery. Based on the physical appearance of the optic nerves, it was clear he had advanced glaucoma, but it required further in-office evaluations.
Accordingly, on this initial visit, multimodal optic nerve images were obtained after dilation. Also, baseline Heidelberg Retina Tomograph (HRT 3) scans and optical coherence tomography (OCT) optic nerve and macular scans were obtained.
The HRT 3 scans confirmed the estimated cup-to-disc ratios seen on physical examination. His OCTs were revealing on several fronts. First, Bruch’s membrane opening (BMO) analysis of both optic nerves demonstrated a thin neuroretinal rim, consistent with advanced disease (Figures 1 and 2).
While reference databases have their role in assessing where your patient stands amongst a reference group of similar but healthy optic nerves, they don’t give a good picture, in details, of how much damage actually exists. They are simply statistical measures of where a patient exists on the bell curve.
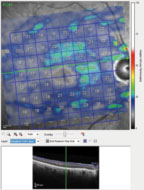 |
| Fig. 3. Note the markedly thin ganglion cell layer in the patient’s right macula. Normal ganglion cell thickness in the macular region varies, but is usually in the 30µm to 40µm range. |
Further examination of the OCT images demonstrated significant loss of ganglion cells in both eyes. The OCT shows the macular scan of the right eye, with segmentation including only the ganglion cells; note the central loss of ganglion cells in the macular region (Figure 3). Additionally, the BMO images seen earlier are an overview of the 12 major meridians around the optic nerve, but closer analysis of the minimum rim width (MRW) of the BMO readings can measure the ganglion cell thickness in the neuroretinal rim along 64 radial OCT scans. The MRW in a sector of the left eye, specifically a sector of the superior temporal neuroretinal rim, shows a ganglion cell thickness of just 65µm (Figure 4). For baseline purposes, both these data points are invaluable in determining further deterioration of the neuroretinal rim or macular ganglion cells, should the disease progress.
At the completion of the initial visit, it was clear that the patient had advanced glaucoma, and important baselines were obtained. But I still was not at the point yet where I could accurately render an opinion as to whether or not the patient should undergo further therapy. I explained that, while he did have advanced glaucoma, more information was needed. Some of that information would need to come from the previous provider, and some would be obtained on the next visit with me.
The patient was scheduled to return to my clinic in one to three weeks for visual field testing, gonioscopy, anterior chamber imaging with both OCT and UBM imaging, as well as an important second IOP reading to get a feel for diurnal variation. At that visit, applanation tensions were 12mm Hg OD and 15mm Hg OS at 10:05am. Threshold standard automated perimetry visual fields demonstrated significant bilateral field defects consistent with the level of glaucomatous damage, with right-field defect-involving fixation. Gonioscopy demonstrated wide-open angles, with mild trabecular pigmentation OU, and the anterior segment OCT scans and the UBMs demonstrated normal anterior chamber anatomy.
Therapies
I was at a disadvantage because I could only see that he had advanced disease, but I could not determine, in two visits, if in fact he was stable or showing progression. Certainly, if a disease is progressing, something else must be done. But if a patient is stable, he could maintain the status quo. At the follow up visit, the patient made it clear he did not want to undergo any procedures.
Given this reluctance, I wondered if we do something to help mitigate his risk of progressing. We could, potentially, change his medications. Given his COPD, we needed to avoid beta blockers, and his current regimen covered the bases.
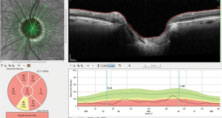 |
| Fig. 4. Note the extreme thinning of the neuroretinal rim as measured from the edge of Bruch’s membrane to the closest portion of the ganglion cells as they enter the optic nerve. In the superior temporal scan, only 65µm of tissue remains. |
Recently, two new medications have become available: Vyzulta (latanoprostene bunod, Bausch + Lomb) and Rhopressa (netarsudil, Aerie). Both facilitate aqueous outflow through the trabecular meshwork, and Vyzulta maintains the prostaglandin-like uveoscleral outflow mechanism common to prostaglandins. I chose to proceed with Vyzulta due to the patient’s insurance and the drug’s performance in the APOLLO Study.1
The challenge was incorporating it into his care. Vyzulta is essentially metabolized into a prostaglandin and nitric oxide. My experience with this medication is that it seems to give a greater reduction in IOP than a prostaglandin alone, owing, most likely, to the effects of the nitric oxide. So, I did something I rarely do—I added this to his current regimen. In other words, I did not stop the Travatan Z that he was currently taking. Here is my logic in doing so (and, yes, this can be criticized!): given he was a fragile glaucoma patient with advanced disease (but the level of frailty was not really known at the time), it would simply be easier to see the effect of the addition of a dual mechanism drug to a regimen that already includes a drug acting via one of the two mechanisms (uveoscleral outflow) in the new drug. Theoretically, there should be no further benefit of the prostaglandin that the Vyzulta generates than to the travoprost; the difference in IOP, if any, could be attributed to the nitric oxide.
When the patient returned in two weeks to see what effect this had on his IOP, applanation tensions were 9mm Hg OD and 10mm Hg OS. IOP readings six days later, after discontinuing Travatan Z, were 10mm Hg OD and OS. Is this enough to stave off further damage? Is this controlling IOP diurnal variations better than the previous combination of medications? Will this keep him from having further intervention?
I don’t know the answer to these questions yet, but I will. Given that he is tolerating the medications well, with what appear to be better IOP reading with less fluctuations (as best we can tell in these few visits), we can at least buy some time before heading to further intervention.
| 1. Weinreb R, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene Bunod 0.024% versus Timolol Maleate 0.5% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The APOLLO Study. Ophthalmol. 2016;123(5):965-73. 1. |

