 |
I recently had the opportunity to again lecture to the New Zealand Association of Optometrists. My last time there, 10 years earlier, therapeutic drug laws had recently been passed and optometrists were going through the education and certification process to obtain prescribing privileges. Now, a decade later, optometrists in New Zealand are registered prescribers (the same as medical doctors) and are allowed to use virtually any medication available within their specialty.
While in New Zealand, my wife and I enjoyed the beauty of the Bay of Islands, the history of the Waitangi Treaty Grounds, and the natural beauty of Fjordland National Park and Milford Sound. We saw unique fauna including the kiwi and the tuatara. However, there were some things that I didn’t see. I did not see anyone dead or dying in the streets. I did not hear the sirens of ambulances racing through the streets, nor did I see any funeral processionals heading to overfilled cemeteries. Indeed, I saw none of these horrendous sights, despite the fact that the most commonly prescribed topical ophthalmic antibiotic in New Zealand is chloramphenicol.
What is Chloramphenicol?
This bacteriostatic antibiotic inhibits bacterial protein synthesis and, when used in high doses or against highly susceptible organisms, it can be bactericidal (in high concentrations).1 Chloramphenicol is available in systemic form as well as a topical ophthalmic 0.5% solution and 1% ointment. Dosing is recommended at five times per day.1
The drug has a broad spectrum of both gram positive and gram negative antibacterial activity.2-6 It is effective in treating anaerobic organisms, mycoplasma, rickettsia and chlamydia.7 More than 95% of Haemophilus influenzae, Neisseria meningitides, Neisseria gonorhoeae, Salmonella typhi, Brucella species and Bordetella pertussis are susceptible to chloramphenicol.7 Especially noteworthy is the low rate of clinical and microbiologic resistance and its ability to conquer organisms that demonstrate resistance to more commonly available antibiotics.8-10
Research shows chloramphenicol is also effective against ocular methicillin-resistant Staphylococcus aureus (MRSA) infections.10 It is used extensively throughout the world (with the exception of the United States) for treatment of acute bacterial infections, corneal trauma and surgical prophylaxis.10-15 Recent resistance studies have shown that chloramphenicol has a strong susceptibility profile and a low resistance.16,17 Notable is that isolates of Streptococcus pneumoniae exhibit non-susceptibility to azithromycin (31%) and penicillin (38%) while remaining susceptible to fluoroquinolones and chloramphenicol.16,17
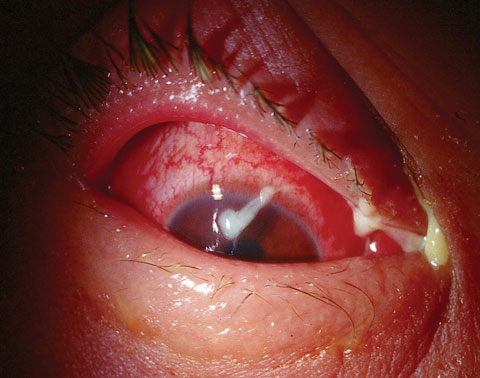 |
| A case of bacterial conjunctivitis, as seen here, is frequently treated with chloramphenicol outside the United States. |
The Fall of Chloramphenicol
Since the inception of systemic chloramphenicol, there has been a reported association with several blood dyscrasias, the most notable of which is aplastic anemia. Fatal aplastic anemia has been associated with systemic chloramphenicol use.1,7,18
The first case of aplastic anemia associated with topical use was reported in the 1960s.7 In 1980 and 1982, the first two cases of fatal aplastic anemia that were thought to be associated with topical chloramphenicol use were reported.19,20 Another fatal case believed to be associated with topical use was reported in 1992.21
To date, there have been 23 cases of aplastic anemia (the majority of which were not fatal) associated with the use of topical chloramphenicol.7 While topical chloramphenicol use is widespread throughout the world, this possible association with aplastic anemia curtailed use of the drug in the United States. In fact, American prescribing information for chloramphenicol issues a warning that it should only be used if there are no other options available.
Another Look
Aplastic anemia is a condition in which bone marrow does not produce sufficient new cells to replenish dying and aging blood cells.22 The marrow suffers from an aplasia that renders it unable to function properly. This results in anemia, with fewer erythrocytes, leukocytes and platelets than normal or fewer than are needed to function properly. Aplastic anemia is life-threatening, demonstrating a 50% mortality rate. The condition can be associated with certain medications or it may occur idiopathically.22
Despite the reported association between chloramphenicol and aplastic anemia, a causal relationship has yet to be conclusively established. Many in the medical community are skeptical of claims that aplastic anemia is actually caused by chloramphenicol.1,7,23-25
Of the 23 possible cases of topical chloramphenicol-induced aplastic anemia, only seven were actually published; the remainder were never fully investigated.7 And of those seven, compelling evidence actually de-emphasized the role of chloramphenicol. For instance, all but one patient had long periods of topical therapy (13 months average), and three patients concurrently used other marrow-toxic medications. Two of these patients had concomitant liver disease. Possible genetic predispositions were also found in three patients (family history of aplastic anemia, pernicious anemia and leukemia).7
During a 10-year span in the United Kingdom, only 11 suspected cases of non-fatal topical chloramphenicol-induced blood dyscrasia were reported, vs. more than 200 million uses.18 This frequency of approximately 1:20 million cases is far less than the 1:100,000 frequency of penicillin-induced anaphylaxis.18 In one report, the incidence of aplastic anemia among users of topical chloramphenicol was 0.36 cases per million weeks of treatment.24 The incidence of idiopathic aplastic anemia among nonusers was 0.04 cases per million weeks.24
Based upon this information, an association between ocular chloramphenicol and aplastic anemia could not be excluded, but the risk was less than one per million treatment courses.24 The minimum total dose of topical chloramphenicol associated with marrow toxicity is 30mg and the minimum duration of exposure is 18 days.1 A typical course of topical chloramphenicol therapy delivers only 10mg to the eye.
Mythbusting
In their editorial based upon the literature and worldwide use, one research team postulated that topical ocular chloramphenicol “possibly” can cause blood dyscrasias and aplastic anemia, and the latter is frequently fatal but is not possible to quantify the risk, which appears to be very rare (approximately one in a million based on a very small number of case reports).26 They also noted that it is likely that there are genetically susceptible individuals to this idiosyncratic reaction, and it is not possible to identify who these individuals are. They remarked that if some medications are not available based on cost alone, drugs like chloramphenicol eye drops could be considered as a viable treatment option.26
The point of this month’s column is not to induce resurgence in chloramphenicol use, but to challenge the myths about this drug. Though difficult to obtain, it is still topically available and can be vital in patients with resistant (including MRSA) ocular infections. By avoiding chloramphenicol use in patients with a family history of aplastic anemia, liver disease or known chloramphenicol sensitivities, this drug could be applied safely and effectively in a wide range of patients.
Chloramphenicol is used extensively in New Zealand, Australia and several other countries. How worried are they about the so-called “deadly effects?” Apparently, not very. Until recently, topical chloramphenicol was available over-the-counter in these countries and any parent could pick up a bottle for a child’s red eye. More recently, it has been moved behind the counter, but is available without a prescription after a brief consultation with a pharmacist (no biomicroscopy required) to ensure that patients didn’t have symptoms of uveitis.
While in New Zealand, I picked up the beginnings of a conjunctivitis myself and foolishly had no samples with me. I went to a local pharmacy where I obtained, not surprisingly, a bottle of chloramphenicol.
Somehow, I survived.
|
1. McGhee CN, Anastas CN. Widespread ocular use of topical chloramphenicol: is there justifiable concern regarding idiosyncratic aplastic anaemia? Br J Ophthalmol. 1996;80(2)82–4. 2. Usha K, Smitha S, Shah N, et al. Spectrum and the susceptibilities of microbial isolates in cases of congenital nasolacrimal duct obstruction. J AAPOS. 2006;10(5):469-72. 3. Matuska S, Rama P, Cavallero A, et al. Nocardia keratitis: a case report. Eur J Ophthalmol. 2006;16(1):164-7. 4. Arantes T, Cavalcanti R, Diniz Mde F, et al. Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Arq Bras Oftalmol. 2006;69(1):33-6. 5. Orden Martínez B, Martínez Ruiz R, Millán Pérez R. Bacterial conjunctivitis: most prevalent pathogens and their antibiotic sensitivity. An Pediatr (Barc). 2004;61(1):32-6. 6. Robert PY, Adenis JP. Comparative review of topical ophthalmic antibacterial preparations. Drugs. 2001;61(2):175-85. 7. Lam RF, Lai JS, Ng JS, et al. Topical chloramphenicol for eye infections. Hong Kong Med J. 2002;8(1):44-7. 8. Egger SF, Ruckhofer J, Alzner E, et al. In vitro susceptibilities to topical antibiotics of bacteria isolated from the surface of clinically symptomatic eyes. Ophthalmic Res. 2001;33(2):117-20. 9. Altaie R, Fahy GT, Cormican M. Failure of Listeria monocytogenes keratitis to respond to topical ofloxacin. Cornea. 2006;25(7):849-50. 10. Cimolai N. Ocular methicillin-resistant staphylococcus aureus infections in a newborn intensive care cohort. American J Ophthalmol. 2006;142(1):183-4. 11. Gordon-Bennett P, Karas A, Flanagan D, et al. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the United Kingdom. Eye (Lond). 2008 May;22(5):620-7. 12. Aslam S, Sheth H, Vaughan A. Emergency management of corneal injuries. Injury. 2007;38(5):594-7. 13. Upadhyay MP, Karmacharya PC, Koirala S, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85(4):388-92. 14. Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9479):37-43. 15. Jassim Al, Khaja KA, Sequeira RR, et al. Trends in ophthalmic antimicrobial utilization pattern in Bahrain between 1993 and 2000: a resurgence of chloramphenicol? Int J Clin Pharmacol Ther. 2003;41(1):36-41. 16. Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. PLoS One. 2018; 17. Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133(12):1445-54. 18. Isenberg SJ. The fall and rise of chloramphenicol. J AAPOS. 2003;7(5):307-8. 19. Fraunfelder F, Bagby G, Kelly D. Fatal aplastic anemia following topical administration of ophthalmic chloramphenicol. Am J Ophthalmol. 1982;93(3):356-60. 20. Abrams S, Degnan TJ, Vinciguerra V. Marrow aplasia following topical application of chloramphenicol eye ointment. Arch Intern Med. 1980;140(4):576-7. 21. McWhae J, Chang J, Lipton J. Drug-induced fatal aplastic anemia following cataract surgery. Can J Ophthalmol. 1992;27(6):313-5. 22. Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:72-7. 23. Rayner SA, Buckley RJ. Ocular chloramphenicol and aplastic anaemia. Is there a link? Drug Saf. 1996;14(5):273-6. 24. Laporte JR, Vidal X, Ballarín E, et al. Possible association between ocular chloramphenicol and aplastic anaemia--the absolute risk is very low. Br J Clin Pharmacol. 1998;46(2):181-4. 25. Field D, Martin D, Witchell L. Ophthalmic chloramphenicol: a review of the literature. Accid Emerg Nurs. 1999;7(1):13-7. 26. Fraunfelder FW, Fraunfelder FT. Restricting topical ocular chloramphenicol eye drop use in the United States. Did we overreact? Am J Ophthalmol. 2013;156(3):420-22. |

