24th Annual Surgery ReportFollow the links below to read other articles from annual update on surgery: The Preoperative Ocular Surface Checkup Understanding the Role of IOL Optics in Postoperative Vision Complaints Excise and Conquer: Adding Minor Surgical Procedures to the Optometric Office |
Laser vision correction (LVC) is the most popular elective medical procedure done in the United States, and its most feared complication is the corneal failure known as ectasia.1 LVC includes Laser In-Situ Keratomileusis (LASIK), Photorefractive Keratectomy (PRK), Small Incision Lenticule Extraction (SMILE), and other less common forms of laser vision correction; all of these surgeries without exception carry a risk of inducing ectasia.2 Optometrists have many of the skills and tools to detect at-risk patients before they ever get to the surgeon and a number of methods and technologies may help reduce the incidence.3,4
This article describes the risk factors for corneal ectasia and how to evaluate patients for them.
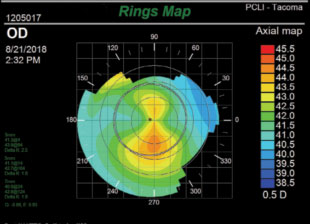 |
| Fig. 1. A subtle case of asymmetric bowtie on topography. Scheimpflug imaging later confirmed that this 18-year-old male had early keratoconus. Notice that the scale is carefully set to 0.5D increments for good sensitivity. |
Post-LVC Ectasia
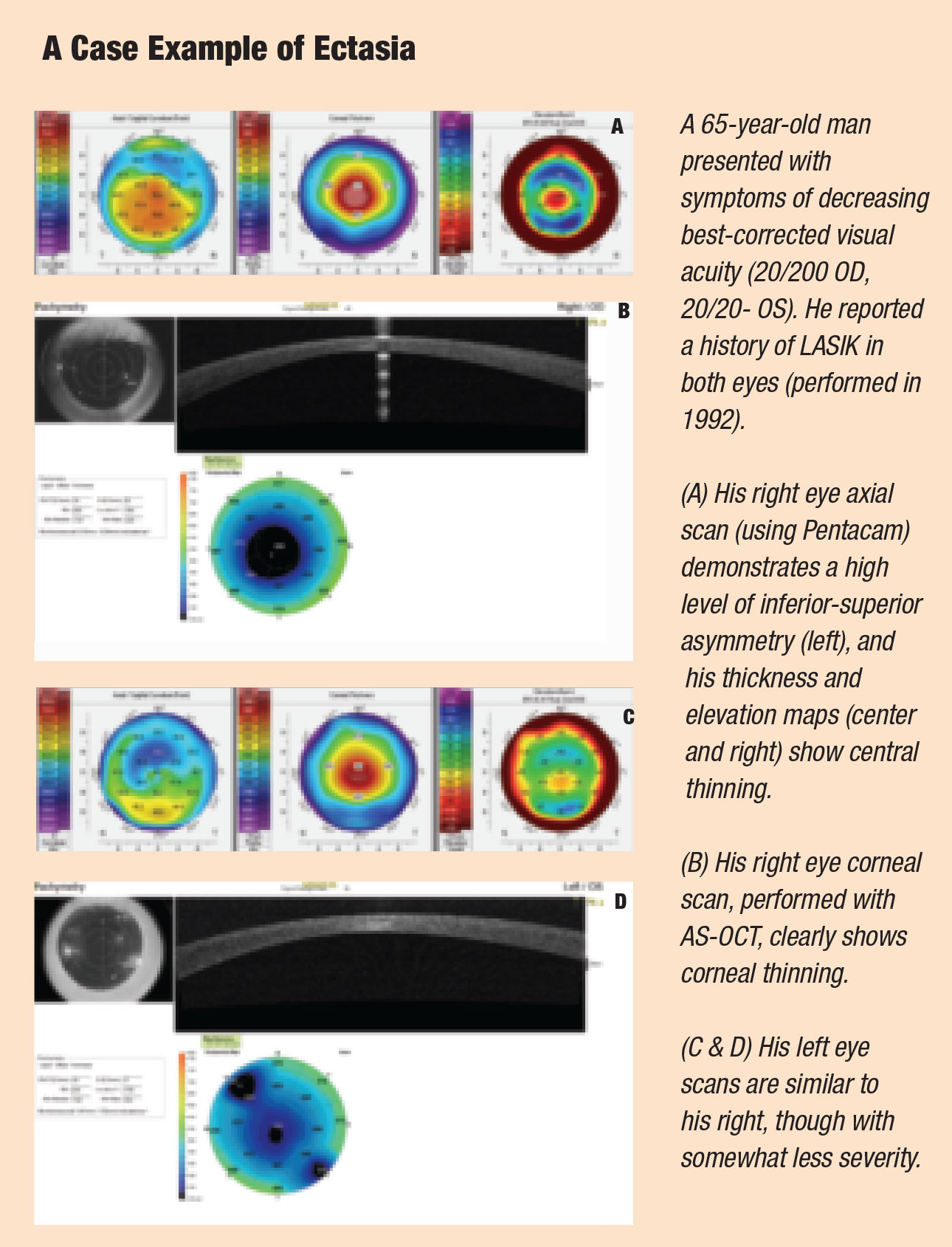
Patients who develop this complication will experience uncorrected visual acuity (UCVA) loss, sometimes severely, and often uncorrectable with glasses.5 The average UCVA in post-LVC patients with ectasia is 20/400, and aberrations reduce even their average best-corrected visual acuity (BCVA) to only 20/100.5
Post-LVC ectasia is a biomechanical failure of the wall of the cornea, resulting in thinning and protrusion, but without acute inflammation.6 After LVC, the flap no longer contributes meaningfully to the strength of the cornea, and the untouched posterior stroma must resist any forces on its own. Within the stroma, intralamellar bonds can fracture and slip under the stress of outside forces, such as intraocular pressure (IOP), eye rubbing, blinking and extraocular muscle forces. Though these forces are small, the damage can mount cumulatively until the cornea finally fails.6 Ectasia can occur from three months to six years after surgery; however, the peak incidence occurs at 12 months.2
 |
Exam Room Clues
Post-LVC ectasia remains such an insidious threat precisely because we have no single test that can determine whether a patient is at risk; instead, we must consider a constellation of risk factors. The primary ones are the patient’s refractive error, age, systemic health, retinoscopy clues, slit lamp findings, pachymetry and especially keratometry.1,2 Because the relative importance of these risk factors has not been definitively quantified, the presence of even one concerning finding is often enough to trigger a decision to avoid surgery.
Certain refractions place a patient at higher risk. In myopic ablations, the higher the correction required, the more stroma the laser must remove; for this reason an ablation of more than -8.00D is considered higher risk, and more than -12.00D is rarely attempted.1 Corneal thickness can affect these calculations to some extent.
Any increasing astigmatism, or astigmatism at 90˚ to that of the fellow eye, increases the risk of an undetected keratoconus.
A stable refractive error over several years is a strong indication that your patient can be considered for LVC. Because refractions tend to stabilize with time, age is a crucial risk factor. A mildly concerning topographic finding can eliminate a candidate at age 20, but be regarded as benign at age 40. Keratoconus is generally detected in the early 20s, and becomes rarer after the fourth decade.1
Certain systemic health conditions pose an absolute contraindication.5 These include:
• Down syndrome (for which one in seven patients develops keratoconus).
• Turner syndrome, a genetic disorder affecting females that among many other effects also predisposes to keratoconus.
• Ehlers-Danlos syndrome, a genetic connective-tissue disorder.
• Marfan syndrome, another genetic connective-tissue disorder.
Any family history of keratoconus should also be considered a red flag. Any scissoring reflex with the retinoscope or oil-drop reflex with the direct ophthalmoscope suggest keratoconus; these would likely be reinforced by other findings such as irregular topography or limited BCVA.
The clinician must be attentive to the classic slit-lamp findings associated with keratoconus. Among these, the most salient is the presence of Vogt’s striae: thin folds on the endothelium, usually vertical. Other possible signs include a Fleischer ring, corneal thinning and prominent corneal nerves.
Any distortion of the manual keratometer mires will probably eliminate a patient from candidacy.
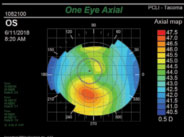 |
| Fig. 2. A difference of greater than 1.4D when comparing equivalent points above and below the horizontal meridian classifies this cornea as high risk due to inferior steepening. |
Enhancements
Because post-LVC ectasia may be subjectively indistinguishable from simple refractive regression, you should be on high alert whenever an LVC patient presents seeking an enhancement. All of the criteria discussed in this article pertain, with a high level of wariness. Keep in mind that post-LVC ectasia does not always manifest as a keratoconus-like inferior steepening and astigmatism, but may only be a suspicious progressive myopia. These cases will be evaluated with great care by any surgery center.
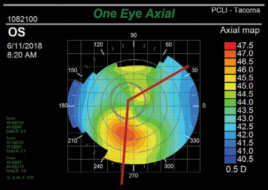 |
| Fig. 3. This analysis of the same cornea seen in Figure 2 demonstrates a skewed radial axis. Note that lines drawn to bisect the superior and inferior lobes deviate from each other by more than 30°. |
Topography
Placido disc topographers (e.g., Medmont, Eyesys, Atlas) use a system of concentric rings projected onto the cornea to calculate corneal curvatures and irregularities. Whether a small-cone or large-cone system is used, these tools are extremely helpful in detecting corneal anomalies.
When scrutinizing topographies for signs of corneas at risk for ectasia, you should be on the alert for these types of topographies: normal, asymmetric bowtie, inferior steepening, skewed radial axis and abnormal.5
• An asymmetric bowtie will show a significantly greater area of steepening inferiorly than superiorly (Figure 1).
• Inferior steepening is defined as a difference of 1.4D or greater when comparing two equivalent points in the inferior and superior cornea (Figure 2).
• A skewed radial axis is interpreted by drawing imaginary lines to bisect the superior and inferior lobes of the bowtie; if these lines deviate from each other by greater than 30°, it is classified as skewed (Figure 3).
• Abnormal topographies include the classic “kissing dove” and “crab claw” patterns that indicate pre-existing corneal failure, as well as anything else out of the ordinary (Figure 4).
If a patient presents with any of the warning signs listed above, order further testing as necessary to determine if they have keratoconus (snowman pattern) or pellucid marginal degeneration (kissing-doves pattern). If in doubt, simply repeat corneal measurements in four to six months, as these cases tend to be progressive.
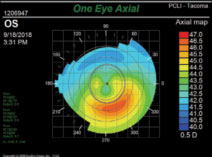 |
| Fig. 4. The “kissing doves” sign clearly indicates early ectasia; this would be classified as abnormal topography. |
Pachymetry
Topography alone does not tell the full story. When topography is combined with pachymetry readings, the clinician is better equipped to educate the patient on their candidacy for LVC.
One of the first risk factors noticed for post-LVC ectasia was an abnormally thin cornea. A central corneal thickness less than 500µm is roughly three standard deviations thinner than average and is abnormal; for years 500µm was considered the cut-off for a safe procedure, and any thinner corneas were considered for PRK. This procedure does not create a flap and the ablation does not go as deep into the stroma as does LASIK.
Any incision weakens the integrity of the cornea. The residual stromal bed (RSB) is the amount of untouched corneal tissue remaining following LVC. Surgeons pay close attention to the depth of the RSB because adhesions between a LASIK flap and virgin cornea are weak, and the flap is considered to no longer contribute any strength to the cornea. Once you obtain a central pachymetry measurement, you can estimate the RSB yourself. If the ablation is done over the standard diameter of 7mm, the depth of the ablation can be estimated using Munnerlyn’s formula.7 For the diopters of correction, use the sphere plus the full astigmatic amount (See “Applying Munnerlyn’s formula”).
An RSB thinner than 300µm increases the patient’s risk. Imprecise corneal pachymetry preoperatively and variability in the depth of the flap will impact the RSB prediction, particularly in those with already thinner than average CCT.8 As more tissue is altered and less virgin tissue remains, the patient’s risk for ectasia increases, even if there are no other predispositions noted.9
Applying Munnerlyn’s FormulaThe formula states: Depth of ablation = optic zone diameter2 x dioptric correction ÷ 3 Assuming a 7mm optic zone, the formula can be restated as 16 x diopters of full correction (sphere + full astigmatism). As the flap can be estimated at 120µm, the RSB is then easily calculated: RSB = pre-op corneal thickness – (120µm flap thickness + depth of ablation) For patient with a 550µm cornea, undergoing standard LASIK for a refraction of -4.00 -2.00 x 180, RSB would be calculated like this: RSB = 550µm – 120µm – (16 x 6.00) = 334µm |
Risk-scoring System
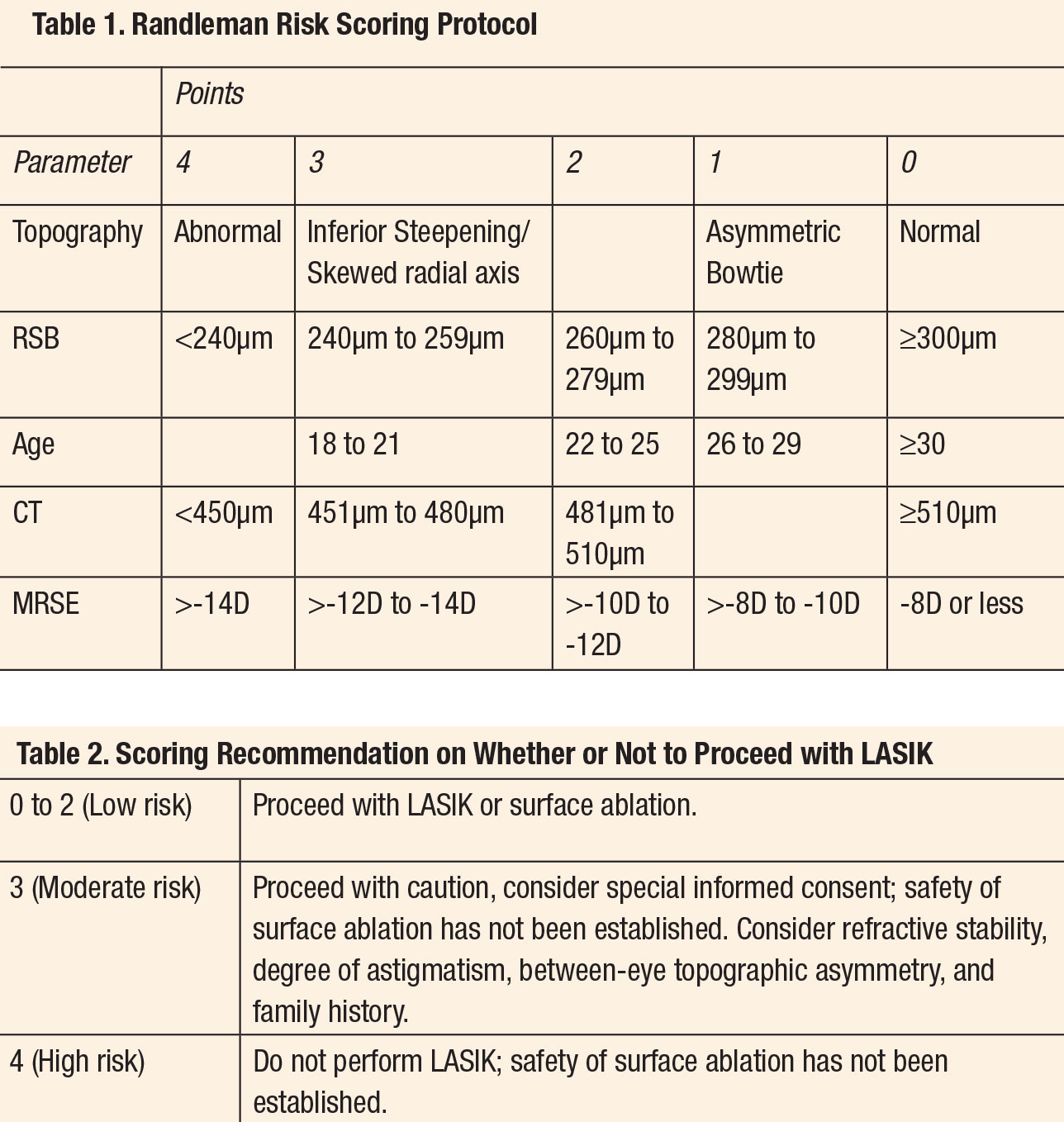
The many risk factors for ectasia are not equally significant. In 2008, Randleman introduced the Ectasia Risk Score System (ERSS), which weights the primary risk factors to help practitioners identify patients who are at the most risk for developing progressive post-keratorefractive ectasia. The system scores the categories of age, residual stromal bed thickness (RSB), preoperative corneal thickness (CT), topography and preoperative spherical equivalent manifest refraction into point intervals by order of risk for ectasia. A summation of those points by category indicates the patient’s predilection for postoperative ectasia with lower points corresponding to lower risk.10The many risk factors for ectasia are not equally significant. In 2008, Randleman introduced the Ectasia Risk Score System (ERSS), which weights the primary risk factors to help practitioners identify patients who are at the most risk for developing progressive post-keratorefractive ectasia. The system scores the categories of age, residual stromal bed thickness (RSB), preoperative corneal thickness (CT), topography and preoperative spherical equivalent manifest refraction into point intervals by order of risk for ectasia. A summation of those points by category indicates the patient’s predilection for postoperative ectasia with lower points corresponding to lower risk.10
The categorization of the topography is the only section requiring the physician’s judgment, but also is weighted heavily as it was the most predictive factor for the eventual development of ectasia (Tables 1 and 2).
Despite a decade of debate, it has proven difficult to create a more robust or efficient scoring system. Any clinician with access to a topographer and pachymeter can complete this assessment independently.
 |
New Approaches
Researchers are currently exploring ways in which subtle clues found in the posterior cornea and in global keratometry may provide more definitive clues of an at-risk cornea. These indications can only be seen when the entire cornea is mapped, including the posterior surface. Devices using slit-scan technology, such as the Orbscan (Bausch + Lomb) and Scheimpflug imaging such as the Pentacam (Oculus) and Galilei (Ziemer Ophthalmic Systems AG) can model the cornea in three dimensions, allowing more detailed measurements of corneal elevation and pachymetry.3,11
The Pentacam’s Belin-Ambrosio and refractive displays focus on specific corneal measurements to alert practitioners when measurements indicate a higher risk for ectasia.12 In addition to detecting anterior corneal anomalies, the Belin-Ambrosio interface calculates both the anterior and posterior corneal surfaces and compares them.12 If the central posterior curvature is significantly steeper than the anterior surface, the patient is more likely to develop ectasia.12 Asymmetry across the horizontal meridian, where the top half does not closely mirror the bottom half, is a strong indicator for an irregular surface not ideal for LVC.13
Undoubtedly, additional factors exist that we have yet to discover. For instance, researchers have shown that eyes with keratoconus have abnormal rebound when deformed, an attribute called corneal hysteresis.13 This quality can now be measured using a device called the Ocular Response Analyzer (Reichert), which indents the cornea with a strong jet of air and then measures its rebound reaction. Some studies suggest that this device will be a helpful tool.14
The prevalence of keratoconus in first-degree relatives is 3.34%, roughly 65 times higher than that in the general population, leading to the search for a genetic marker for ectasia risk.15 This has been complicated, but recent genome-wide linkage studies have made significant findings. Several autosomal-recessive genes have now been implicated, including genes coding for collagens and the production of extracellular matrix. One of the most significant of these is the LOX gene, which codes for a copper-dependent enzyme responsible for the crosslinking of collagens and elastin; defects in this gene have also been linked with a predisposition to thoracic aortic aneurysms and dissections.16
Unfortunately, despite the most rigorous preoperative screening, some patients will still go on to develop visually significant ectasia. Research shows 32% of post-LVC ectasia cases have completely normal preoperative topographies, with no warning signs even in post-hoc analysis.8 For this reason it is crucial to be clear with each LVC candidate that we cannot eliminate all risk, and that these elective procedures inevitably carry some risk of ectasia, as well as the other risks, such as glare, dry eyes, inaccurate outcome, inflammation, epithelial ingrowth and hastening of presbyopia. Carefully explain this to your patient, listen until they explicitly accept this risk and document the conversation in your chart.
Drs. Kuhn-Wilken and Roan are staff optometrists at Pacific Cataract & Laser Institute, in Tacoma, WA and Bellevue, WA, respectively.
1. Saad A, Binder P, Gatinel D. Evaluation of the percentage tissue altered as a risk factor for developing post-laser in situ keratomileusis ectasia. J Cataract Refract Surg. 2017;43:946-51. 2. Wolle M, Randleman J, Woodward M. Complications of refractive surgery: ectasia after refractive surgery. Int Ophthalmol Clin. 2016;56(2):129-41. 3. Woodward M, Randleman J, Russell B, et al. Visual rehabilitation and outcomes for ectasia after corneal refractive surgery. J Cataract Refract Surg. 2008;34(3):383-8. 4. Binder P, Trattler W. Evaluation of a risk factor scoring system for corneal ectasia after LASIK in eyes with normal topography. J Refractive Surg. 2010; 26(4):241-50. 5. Spadea L, Cantera E, Cortes M, et al. Corneal ectasia after myopic laser in situ keratomileusis: a long-term study. Clinical Ophthalmology. 2012:6(1): 1801-13. 6. Chan C, Saad A, Randleman J. Analysis of cases and accuracy of 3 risk scoring systems in predicting ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2018;44:979-92. 7. Wang M. Keratoconus & keratoectasia: prevention, diagnosis, and treatment. Thorofare NJ: SLACK Inc. 2010;51-60. 8. Binder P, Lindstrom R, Stulting R, et al. Keratoconus and corneal ectasia after LASIK. J Cataract Refract Surg.2005;31(11):2035-8. 9. Chang A, Tsang A, Contreras J, et al. Corneal tissue ablation depth and the Munnerlyn formula. J Cataract Refract Surg. 2003 Jun;29(6):1204-10. 10. Randleman JB, Woodward M, Lynn MJ, et al. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37-50 11. Ambrósio R, Ramos I, Lopes B, et al. Assessing ectasia susceptibility prior to LASIK: the role of age and residual stromal bed (RSB) in conjunction to Belin-Ambrósio deviation index (BAD-D). Revista Brasileira De Oftalmologia, 2014;73(2):87-95. 12. Belin MW, Khachikian SS, Ambrósio Jr R, Salomão M. Keratoconus/ectasia detection with the Oculus Pentacam: Belin/Ambrósio Enhanced Ectasia Display. Highlights of Ophthalmology. 35:6;5-12. 13. Belin M, Holladay J, Michelson M, et al. The Pentacam: precision, confidence, results, and accurate Ks! Cataract and Refract Surg Today. www.pentacam.com/fileadmin/user_upload/pentacam.de/downloads/publikationen/sonderdrucke/2007-Supplement_Pentacam_AAO_2006.pdf. January 2007. Accessed November 17, 2018. 14. Galletti JG, Pförtner T, Bonthoux FF. Improved keratoconus detection by ocular response analyser testing after consideration of corneal thickness as a confounding factor. J Refract Surg. 2012;28:2025-208. 15. Wang Y, Rabinowitz YS, Rotter JI, et al. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J of Medical Genetics. 2000; 93:403-409. 16. Bykhovskaya Y, Margines B, Rabinowitz YS. Genetics in keratoconus: where are we? Eye and Vision. 2016:3;16. |

