Floppy eyelid syndrome (FES) is a condition characterized by an elastic-like upper eyelid that is easily pliant and everted with minimal lateral traction (Figure 1).1 The tarsal plate is found to be “rubbery,” with loss of the rigidity that normally maintains the integrity of the eyelid.1 This clinical finding is now linked to several more severe ocular and systemic conditions, most notably keratoconus and obstructive sleep apnea-hypopnea syndrome (OSAHS), which we will discuss in further detail.1-9
FES Research
In the study that originally described the condition, a group of 11 men displayed the triad of obesity (BMI>30); lax, easily-malleable upper eyelid; and tarsal papillary conjunctivitis of the upper eyelid palpebral conjunctiva. Their eyelids were found to spontaneously evert during sleep, and as a result of exposure of the upper palpebral conjunctiva to the pillow, these patients developed chronic conjunctivitis. The affected side corresponded to the side that the patient usually slept on, and if bilateral, the patient either slept without a preferred side or slept on their stomaches. Another study found asymmetric involvement in 50% of patients and an equal distribution between either eye in the remainder of patients.2 Later studies found that up to 37% of cases are female and that not all patients with FES are overweight or obese.3,4,10 In fact, several case reports document FES in women, children, infants and non-obese patients, although overweight men still represent the majority of patients with FES.5
Clinical Presentation
The symptoms include a nonspecific irritation, foreign body sensation, mucoid discharge, dryness, redness, photosensitivity and eyelid swelling.7,8,11,12 Unfortunately, due to the similarity in symptoms, patients are often misdiagnosed with chronic infective conjunctivitis, blepharitis or dry eye syndrome, which may lead to delayed or missed treatment for months or even years.11,12 The goal of the clinician is to recognize the specific traits of FES that differentiate it from inflammation of the eyelids and ocular surface conditions, as FES does not respond to standard anti-inflammatory therapy. Earlier detection will not only relieve the patient of discomfort but will also stop the progression toward more severe ocular sequelae if improperly managed.
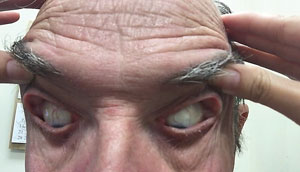 | |
| Fig. 1. This patient’s elastic, easily pliant eyelids prompt a diagnosis of FES, which is also linked to several severe ocular and systemic diseases. |
Eyelid changes that may accompany FES include blepharoconjunctivitis, blepharoptosis, lower lid ectropion, hyperkeratotic skin lesion of the lids and face, keratinization of the conjunctival surface of the affected upper eyelid, upper eyelid ptosis, eyelash ptosis and loss of eyelash parallelism.2,6,13
Changes in tarsus integrity and redundancy of the eyelid can affect the stability of the tissue into which the cilia are anchored, causing downward displacement of the hairs, referred to as eyelash ptosis.7,12,13 Abnormalities of the levator muscle are not indicated as a cause of symptoms. As previously mentioned, lash ptosis is a prominent clinical finding in FES where lashes are directed downward as a result of eyelid laxity.7,13 In severe cases, trichiasis is seen with subsequent corneal abrasions.13 All these factors have the potential to develop into corneal ulceration, scarring, vascularization with pannus and, rarely, corneal perforation.1,3,13,14 Loss of elasticity, sagging and wrinkling of the facial skin on the same side as the FES are also often seen, giving the patient the appearance of premature aging or palsy on the contralateral side of the face.7 In addition to laxity of the adnexal tissue and lids themselves, FES affects the eye in several other ways.
Corneal complications in FES often stem from multifactorial processes.3,8,12 Superficial punctate keratopathy is nearly always present in FES due to direct trauma to the corneal surface as a result of everted lids during sleep.8,12 Involvement of the upper part of the cornea differentiates FES from nocturnal lagophthalmos, which tends to compromise the inferior third of the cornea. In addition, poor upper lid apposition to the globe can lead to poor ocular surface wetting and subsequent epitheliopathy. Furthermore, inflammation and abnormalities of the tarsal and accessory lacrimal gland, as a result of FES, can lead to decreased meibomian gland secretions. Dry eye syndrome and blepharitis are often a secondary manifestation of FES and may easily be assumed the primary cause of symptoms.12 Though treatment of these two presentations may alleviate some of the patient’s symptoms, it is not sufficient in addressing the primary cause. Several cases of ipsilateral keratoconus (KCN) have also been associated with FES.3,12,13 One particular case noted that both conditions were isolated to the side on which the patient would sleep.15
Etiology
Unfortunately, the exact cause of FES is still unknown, but several theories exist. Several authors believe poor lid apposition between the lax eyelid and globe results in mechanical conjunctival irritation.1,12,16,17 These studies show cases of patients sleeping on the affected side and suggest that chronic eyelid eversion may cause mechanical trauma to the tarsus.1,9,12,15,16,18 The mechanical stress theory is further supported by the link between FES and KCN, as eye rubbing and presentation ipsilateral to the side on which the patient sleeps, are associated.12,15,19,20 One study found a 7% prevalence of KCN in association with FES, which is significantly higher than in the general population (~0.004% to 0.6%).20 To better understand the pathophysiology, researchers looked toward histopathology for answers.
Several histopathological studies demonstrate depleted levels of elastin within the tarsal plate in patients with FES relative to normal lids rather than changes in lid collagen between FES patients and controls.14
Researchers also noted reduced levels of elastin in the tarsal connective tissue, eyelid skin and ciliary roots.18 The elastin fibers were structurally flawed and described as “moth-eaten.” The same group’s immunohistological findings revealed increased matrix metalloproteinase (MMP) enzymes, particularly MMP-7 and MMP-9, in areas without inflammatory involvement.18 These findings were later corroborated by other groups as well.14,18 MMP-7 and MMP-9 are specific enzymes that only control elastin degradation and have no effect on collagen fibers. A study found repeated mechanical trauma as the source of increased activity in these elastolytic enzymes.16
In studying the presence of the MMP-7 and MMP-9 elastolytic enzymes, another group found elevated leptin levels in proportion to body fat content.21 Leptin is a hormone regulated by adipose cells shown to increase MMP expression and activity. Therefore, a cascade results in which an increase in leptin secretion causes increased activation of MMP-7 and MMP-9, which go on to damage elastin fibers of the tarsal plate in many of the obese patient population. Ischemia-reperfusion injury has also been proposed as a cause due to reports that it may also lead to upregulation of MMPs.3 Interestingly, elevated MMP levels have also been noted in KCN cases, suggesting that FES and KCN have a partially shared underlying pathological mechanism that warrants further investigation.12,15
FES Comorbidities
Several systemic conditions are linked to FES findings, most commonly obesity (43%), hypertension (13%), diabetes mellitus (12%) and obstructive sleep apnea (85%).1,3,5,7 The latter three conditions are common in patients who are obese and may not represent independent risk factors.19 One of the most consistently reported associations is obstructive sleep apnea-hypopnea syndrome (OSAHS).6-8,13,19,20,22 Investigators found the prevalence of OSAHS in patients with strictly defined FES higher than in the general population.20 Although only 2% to 16% of OSAHS patients suffer from FES, the majority of patients with FES do suffer from symptoms of OSAHS.5,7,9 FES is often seen as an indicator of severe OSAHS.5,8 In addition to daytime somnolence, OSAHS carries significant risks of morbidity, with a high reported incidence of cardiovascular and cerebral sequelae.8,23
Preoperative FES
It may serve as an additional motivation for patients to get evaluated for OSAHS in a sleep study to tell them that early detection and management of FES will decrease contraindications and complications associated with anterior segment surgeries, such as cataract extraction and refractive correction. Blepharitis and keratoconjunctivitis, commonly associated findings in FES, left untreated preoperatively make patients more susceptible to postoperative endophthalmitis.6,13,24 In addition, the patient will have to wear a shield during the entire healing process to prevent spontaneous lid eversion, which could lead to direct trauma to the incision wound and the already compromised corneal surface.
Blepharoptosis after cataract surgery has long been documented.25 Trauma to the levator muscle from anesthetic injection, use of superior rectus bridle sutures, postoperative lid edema or prolonged patching may result in ptosis.25-28 Many studies suggest the cause of blepharoptosis is multifactorial.25,26,28 However, the use of only topical anesthetics in refractive surgery suggests that the rigid eye speculum may strongly contribute to developing postoperative lid laxity.
For refractive and cataract surgery to be performed safely and accurately, some form of lid retraction is necessary. In most cases, ptosis will improve with time, but in cases of an already compromised tarsal plate, healing may take much longer, or not occur at all. The most common form of postoperative ptosis is aponeurotic, meaning there is dehiscence or disinsertion of the levator aponeurosis from the tarsus.25,27 Patients with FES have an already weakened tarsal plate, further increasing the risk of developing postoperative ptosis. Complaints of dry eye will likely worsen after any anterior segment operation, and a poorly apposed lid will not allow complete and consistent lubrication across the ocular surface.
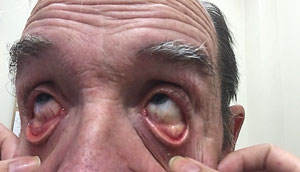 | |
| Among other issues, floppy eyelid syndrome is frequently associated with superficial punctate keratopathy due to direct trauma to the corneal surface from everted lids during sleep. |
Surgical Treatment Options
Surgically, tightening the eyelids can reduce symptoms and signs of FES, as well as preserve ocular surface integrity.29 Surgical intervention options include: full-thickness wedge excision, lateral tarsal strip, lateral canthal tendon plication and lateral tarsorrhaphy. The procedure can involve full-thickness, horizontal resection and shortening of the loose redundant tissue or tightening of the canthal tendons to improve lid apposition to the globe.7,8 The goal is to prevent further spontaneous eversion of the lid during sleep. Studies show this surgery, in conjuction with management of OSAHS, may decrease instances of recurring FES.7,8
Patients with FES may benefit from sleep studies to determine if they have OSAHS. The eye care provider should notify the patient’s primary physician about the findings and their associated systemic concerns. Then either the eye care provider or the primary care physician can refer the patient for a polysomnography, also known as a sleep study.30 Patients should be prepared to stay overnight at these sleep centers where their brain activity, eye movement, heart rate, blood pressure, blood oxygen levels and volume of air inhaled and exhaled are recorded.30 If the patient is indeed diagnosed with OSAHS, the primary care physician may schedule a continuous positive airway pressure (CPAP) titration where a technician will adjust the airflow through the CPAP machine to find the most optimal settings while the patient sleeps.30 Since OSAHS involves shallow or pauses in breathing, the CPAP machine uses mild air pressure to maintain an open airway at night. Some patients may be required to return to study centers several times before the optimal settings for their CPAP machine is determined.30
Direct management of patients’ systemic condition has proven to greatly reduce symptoms; in fact, CPAP treatment alone has shown to improve symptoms of both OSAHS and FES.22 Patients with FES who were not treated for their OSAHS may actually develop recurrent signs of FES months or years after surgical tightening of their eyelids.7 By screening for patients who are at potential risk for OSAHS, you can maximize efficacy of medical and surgical treatments. Close comanagement with the operating ophthalmologist is important to guarantee best possible outcomes for any surgeries in or around the globe.
Non-Surgical Treatment Options
Conservative methods of treatment include shielding, patching or taping the eyes at night. These options do nothing to address the root cause of symptoms, but mechanically protecting the eyes at night often improves symptoms and reduces incidence of the suspected mechanical trauma associated with the papillary conjunctival change along with punctate keratopathy.
Sleep History Questionnaire
If a patient were to answer yes to any of the above questions, it may suggest an issue with sleep-disturbed breathing. Careful evaluation of eyelids, eyelashes and adnexa of patients with persistent ocular surface complaints that are nonresponsive to topical lubrication is strongly recommended. |
However, patients may report discomfort with these treatments, and compliance is subsequently affected. Tear substitutes, gels and bland ointments are only temporary treatments and only treat the dry eye component of the condition. The ester-based steroid loteprednol etabonate is also an alternative management of ocular surface and lid inflammation in FES but may be financially challenging for patients to stay on long-term.
In cases when ketone-based topical steroid drops are used to treat ocular surface inflammation, the increased risks of developing glaucoma, early cataracts and ocular infections are still a concern.
Close examination of the laxity, appearance and position of the tarsus should become a routine part of each dry eye and anterior segment evaluation prior to surgery.
Although floppy eyelid syndrome itself is not life threatening, it can lead to serious sight-threatening conditions and be an indicator of fatal systemic conditions. Poor response to usual dry eye treatments or a positive response to sleep history questions or both, could indicate OSAHS, which is linked to morbid systemic conditions. FES is therefore predictive of OSAHS and should signify the primary eye care provider to consider a referral to evaluate the sleep habits of presenting patients.
Dr. Roan graduated from the University of Missouri-St.Louis, College of Optometry and completed her residency at the Jonathan M. Wainwright VAMC.
Dr. Roan would like to acknowledge Aaron Bronner, OD, FAAO, Jodi Moore, OD, Jennifer Melsness, OD, Haley McCreery OD, and Gleb Sukhovolskiy, OD, FAAO for providing valuable feedback and support.
2. Netland P, Sugrue S, Albert D, Shore J. Histopathologic features of the floppy eyelid syndrome. Ophthalmology. 1994 Jan;101(1):174-81.
3. Culbertson W, Tseng S. Corneal disorders in floppy eyelid syndrome. Cornea. 1994;13:33-42.
4. Paciuc M, Mier M. A woman with the floppy eyelid syndrome. Am J Ophthalmol. 1982;93:255-6.
5. Muniesa M, Huerva V, Sanchex-De-La-Torre M, et al. The relationship between floppy eyelid syndrome and obstructive sleep apnoea. Br J Ophthal. 2013 Nov;97(11):1387-90.
6. Woog J. Obstructive sleep apnea and floppy eyelid syndrome. Am J Ophthalmol. 1990:110: 223-225.
7. Mcnab A. The eye and sleep apnea. Sleep Med Rev. 2007 Aug;11(4):269-76.
8. Mcnab A. Floppy eyelid syndrome and obstructive sleep apnea. Ophthal Plast Reconstr Surg. 1997 Jun;13(2):98-114.
9. Karger R, White W, Park W, et al. Prevalence of floppy eyelid syndrome in obstructive sleep apnea-hypopnea syndrome. Ophthalmology. 2006;113:1669-74.
10. Rossiter J, Ellingham R, Hakin K, Twomey J. Corneal melt and perforation secondary to floppy eyelid syndrome in the presence of rheumatoid arthritis. Br J Ophthalmol. 2002;86:483.
11. Abdal H, Pizzimenti J, Purvis C. The eye in sleep apnea syndrome. Sleep Med. 2006;7(2):107–115.
12. Ezra D, Beaconsfield M, Collin R. Floppy eyelid syndrome: stretching the limits. Surv Ophthalmol. 2010 Jan-Feb;55(1):35-46.
13. Langford J, Linberg J. A new physical finding in floppy eyelid syndrome. Ophthalmology. 1998 Jan;105(1):165-9.
14. Netland P, Sugrue S, Albert D, Shore J. Histopathologic features of floppy eyelid syndrome. Involvement of tarsal elastin. Ophthalmology. 1994:101:174-81.
15. Donnenfeld E, Perry H, Gibralter R, et al. Keratoconus Associated with Floppy Eyelld Syndrome. Ophthalmology. 1991 Nov;98(11):1674-8.
16. Swartz M, Tschumperlin D, Kamm R, Drazen J. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci USA. 2001;98:6180-5.
17. Liu D, Di Pascuale M, Sawai J. Tear film dynamics in floppy eyelid syndrome. Invest Ophthalmol Vis Sci. 2005 Apr;46(4):1188-94.
18. Schöltzer-Schrehardt U, Stojkovic M, Hofmann-Rummelt C,et al. The pathogenesis of floppy eyelid syndrome. Involvement of matrix metallopoteinases in elastic fiber degradation. Ophthalmology. 2005;112:694-704.
19. Ezra D, Beaconsfield M, Sira M, et al. The associations of floppy eyelid syndrome: A case control study. Ophthalmology. 2010 Apr;117(4):831-8.
20. Fowler A, Dutton J. Floppy eyelid syndrome as a subset of lax eyelid conditions: relationships and clinical relevance (an ASOPRS thesis). Ophthal Plast Reconstr Surg. 2010 May-Jun;26(3):195-204.
21. Taban M, Perry J. Plasma Leptin levels in patients with floppy eyelid syndrome. Ophthal Plast Recontr Surg. 2006;22:375-7.
22. McNab A. Reversal of floppy eyelid syndrome with treatment of obstructive sleep apnoea. Clin Experiment Ophthalmol. 2000; 28:125-6.
23. Hashim S, Al Mansouri F, Farouk M, et al. Prevalence of glaucoma in patients with moderate to severe obstructive sleep apnea: ocular morbidity and outcomes in a 3 year follow-up study. Eye (Lond). 2014 Nov;28(11):1304-9.
24. Asadolah M, Djalilian A. Cataract surgery in the face of ocular surface disease. Current Opinion in Ophthalmology. 2012;23:68–72.
25. Paris G, Quickert M. Disinsertion of the aponeurosis of the levator palpebrae superioris muscle after cataract extraction. Am J Ophthalmol. 1976;81:337-40.
26. Kuo I. The effect of ptosis on cataract surgical planning. Case Rep Ophthalmol. 2015 Apr;6(1):132-8.
27. Deady J, Price N, Sutton G. Ptosis following cataract and trabeculectomy surgery. Br J Ophthal. 1989 Apr;73(4):283-5.
28. Singh S, Sekhar G, Gupta S. Etiology of ptosis after cataract surgery. J Cataract Refract Surg. 1997 Nov;23(9):1409-13.
29. Ezra D, Beaconsfield M, Sira M, et al. Long-term Outcomes of Surgical Approaches to the Treatment of Floppy Eyelid Syndrome. Ophthalmology. 2010 Apr;117(4):839-46.
30. Types of Sleep Studies. NHLBI, NIH. www.nhlbi.nih.gov/health/health-topics/topics/slpst/types. Accessed Sept. 10, 2015.

