 |
A 58-year-old man presented urgently after noticing a painless visual change in his left eye when he awoke the previous morning. He knew that something was off, but initially wasn’t too concerned because he still had good central vision. He didn’t become concerned until the next day when the visual sensation didn’t improve.
He had a history of treated hypertension. His visual acuity was 20/25 OS, but there was a relative afferent pupil defect (RAPD) in that eye. He had an inferiorly located defect on confrontation visual fields, and threshold perimetry revealed a significant inferior arcuate defect. A dilated retinal examination revealed an edematous, hyperemic optic disc in the left eye and a small optic disc in the right with virtually no optic cup.
Diagnosis
Based upon the history of abrupt painless vision loss, an inferiorly located visual field defect, a hyperemic edematous optic disc and a classic “disc at risk” in the fellow eye, he was diagnosed with non-arteritic anterior ischemic optic neuropathy (NAION).
NAION typically presents as a painless, unilateral disturbance of vision. Patients are generally 55 to 65 years old; however, the onset of NAION may occur as early as the patient’s late 30s.1 Men and women are affected equally, and the disease is most prevalent in Caucasians.1,2 Many patients with NAION have some underlying systemic disease, although they may not be aware of any health problems at the time of presentation.3 Most often, vascular disorders such as hypertension, diabetes, atherosclerosis or a combination are present.3
Vision loss is not always abrupt. Some patients report a rapid decline in acuity over several days, while 45% of patients worsen over two weeks, with another 29% reporting visual deterioration over 30 days.4 Visual acuity may be moderate to poor; about half of patients present with 20/60 or better, while roughly a third have entering acuity of less than 20/200.2 Visual field defects most commonly include inferior altitudinal, inferior arcuate, inferior nasal and cecocentral scotomas.1
Examination of these patients reveals an RAPD in the involved eye. They generally experience little pain or other associated symptoms, a feature that helps to distinguish this condition from other optic neuropathies. The involved optic disc will be edematous and hyperemic.1,5
A key diagnostic characteristic of NAION involves a small, crowded optic disc with minimal cupping in the contralateral eye. This “disc at risk,” as some have called it, is recognized as a significant risk factor for the development of NAION in predisposed individuals.1,6,7 NAION represents an infarction of the anterior portion of the optic nerve, typically involving the paraoptic branches of the short posterior ciliary arteries.7,8
While no specific laboratory studies can confirm a diagnosis of NAION, an erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) should be ordered for any older patient for whom the diagnosis is uncertain and the possibility of giant cell arteritis exists.
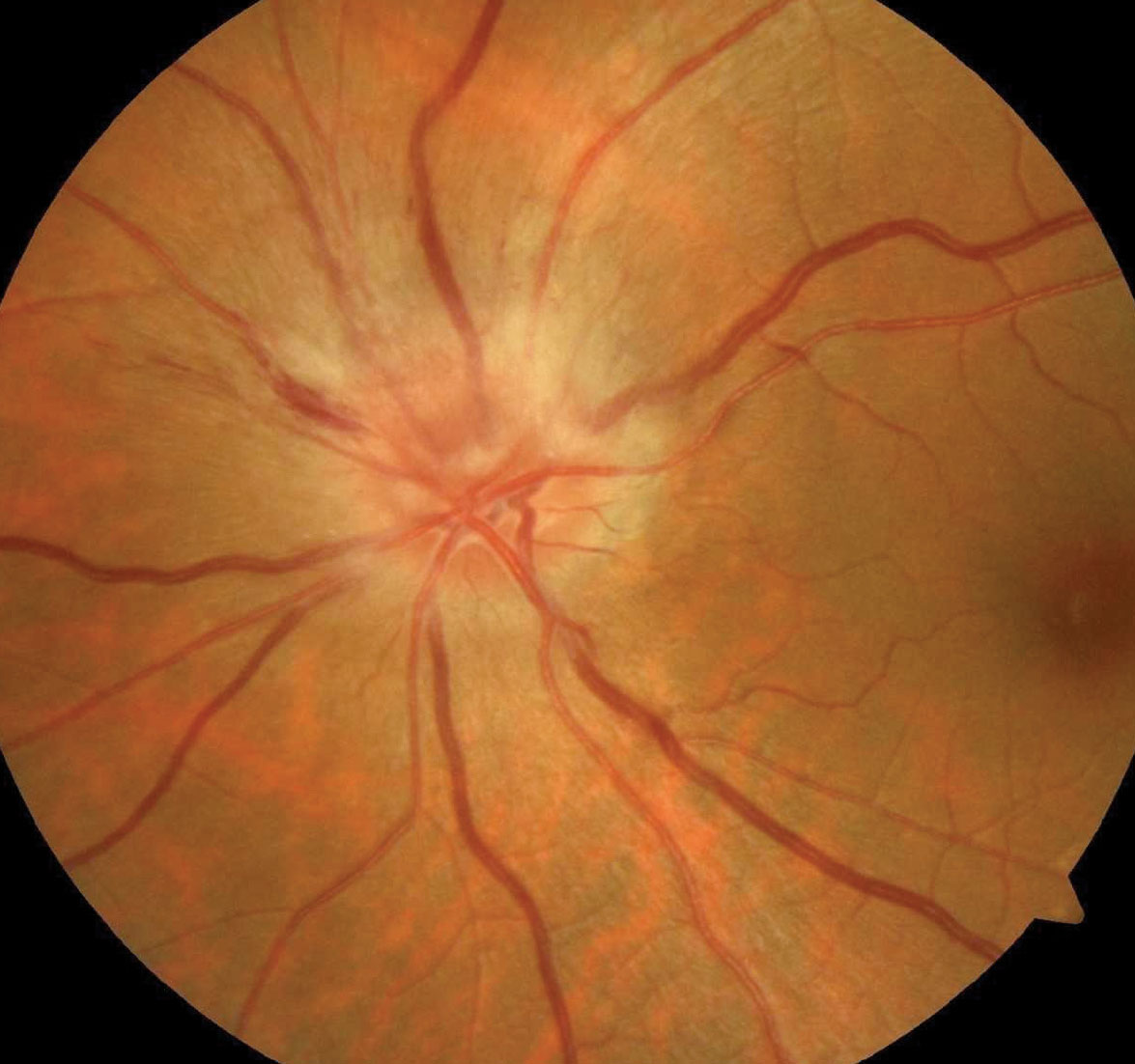 |
| A patient with NAION. Researchers are hunting for a treatment. Photo: Neil Miller, MD. Click image to enlarge. |
Treatment Options
No universally accepted treatment for NAION yet exists. NAION eyes can spontaneously recover some visual function.9 Optic nerve sheath fenestration was investigated in the early 1990s, but abandoned as a therapy because of poor efficacy and high risk.9 Daily aspirin therapy has been recommended as prophylactic therapy, but the five-year cumulative benefit was less than 3%, according to researchers.10
Intravitreal injections of ranibizumab and erythropoietin individually have been noted to increase visual function in eyes with NAION, but again these were very small case series without controls.11,12 Research shows intravitreal injections of triamcinolone acetonide (IVTA) can improve visual function in eyes with NAION.13-16 However, most of that research was from case reports with no control group. Larger, controlled studies are needed before IVTA can be considered an effective therapy.
Topical brimonidine tartrate 0.2% has been anecdotally used based upon presumed neuroprotection and ease and availability of use. A double-masked, placebo-controlled, randomized multicenter trial involving brimonidine showed that visual acuity did not change to a statistically significant degree with treatment, though some non-significant trends for better visual field results were seen in the brimonidine group. While safe, a statistically significant advantage for the patients receiving brimonidine tartrate could not be shown.17 Systemic steroids have also been well demonstrated to have no recognizable benefit for visual function in NAION and, at high doses, have caused significant systemic adverse effects.17-20
All May Not Be Lost
Though traditional and non-traditional approaches show little to no benefit for patients with NAION, a study is currently enrolling patients in an effort to bring an effective treatment to market. Quark Pharmaceuticals, in concert with the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC), is conducting a randomized, double-masked, sham-controlled trial of QPI-1007 delivered by single or multi-dose intravitreal injection(s) to patients with acute NAION. This efficacy and safety study will enroll approximately 800 subjects with recent-onset NAION. Subjects will be randomized into one of three groups in a 1:1:1 ratio, and assigned to receive QPI-1007, a sham procedure or both. Subjects will have a 66% chance of receiving active treatment and a 33% chance of receiving a sham placebo procedure. Total study time involvement will be approximately 12 months. This study will determine the effect of QPI-1007 on visual function in subjects with recent-onset NAION and assess the safety and tolerability of intravitreal injections of QPI-1007 in this population. This study will also evaluate the structural changes in the retina following administration of QPI-1007. Criteria assessed from day one through month 12 will be final mean change in best-corrected visual acuity and visual field.
Study enrollment criteria involves males and females ages 50 to 80 years old, and a positive diagnosis of first episode of NAION in the study eye with symptom onset within 14 days. Key exclusion criteria include any treatment for the current episode of NAION, including systemic steroids or brimonidine, use of drugs known to cause optic nerve or retinal toxicity such as chloroquine, hydroxychloroquine, ethambutol or vigabatrin, as well as clinical evidence of temporal arteritis.
Optometrists can help these patients by referring potential patient-subjects to a clinical study site across the United States. If you suspect your patient has NAION, consider referring as soon as possible. Early referral is critical, as study enrollment is limited to 14 days from onset of symptoms. Study sites will complete full diagnosis, determine eligibility and provide all study details. You can identify and contact a study site by visiting www.eyeactnow.com for study information and to locate a study site. You can also visit www.clinicaltrials.gov for more information and to identify current sites. Your involvement may shed light on a possible treatment for a condition that has eluded management.
1. Gray LG. NAION: Diagnosing in the negative. Rev Optom. 2000;137(6):83-98. 2. Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol. 1994;14(1):38-44. 3. Giambene B, Sodi A, Sofi F, et al. Evaluation of traditional and emerging cardiovascular risk factors in patients with non-arteritic anterior ischemic optic neuropathy: a case-control study. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):693-7. 4. Ischemic Optic Neuropathy Decompression Trial Study Group. Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the ischemic optic neuropathy decompression trial. Arch Ophthalmol. 1996;114(11):1366-74. 5. Hayreh SS. Anterior ischemic optic neuropathy. Differentiation of arteritic from non-arteritic type and its management. Eye. 1990;4(Part 1):25-41. 6. Burde RM. Optic disc risk factors for nonarteritic ischemic optic neuropathy. Am J Ophthalmol. 1993;116(6):759-64. 7. Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003; 23(2):157-63. 8. Collignon-Robe NJ, Feke GT, Rizzo JF. Optic nerve head circulation in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Ophthalmology. 2004;111(9):1663-72. 9. The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273(8):625-32. 10. Beck RW, Hayreh SS, Podhajsky PA, et al. Aspirin therapy in nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123(2):212-7. 11. Bajin MS, Selver OB, Taskin O, et al. Single intravitreal ranibizumab injection in eyes with acute non-arteritic anterior ischaemic optic neuropathy. Clin Exp Optom. 2011;94(4):367-70. 12. Modarres M, Falavarjani KG, Nazari H, et al. Intravitreal erythropoietin injection for the treatment of non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2011;95(7):992-5. 13. Yaman A, Selver OB, Saatci AO, Soylev MF. Intravitreal triamcinolone acetonide injection for acute non-arteritic anterior ischaemic optic neuropathy. Clin Exp Optom. 2008;91(6):561-4. 14. Jonas JB, Spandau UH, Harder B, Sauder G. Intravitreal triamcinolone acetonide for treatment of acute nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):749-50. 15. Sohn BJ, Chun BY, Kwon JY. The effect of an intravitreal triamcinolone acetonide injection for acute nonarteritic anterior ischemic optic neuropathy. Korean J Ophthalmol. 2009;23(1):59-61. 16. Kaderli B, Avci R, Yucel A, et al. Intravitreal triamcinolone improves recovery of visual acuity in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2007;27(3):164-8. 17. Wilhelm B, Lüdtke H, Wilhelm H; BRAION Study Group. Efficacy and tolerability of 0.2% brimonidine tartrate for the treatment of acute non-arteriticanterior ischemic optic neuropathy (NAION): a 3-month, double-masked, randomised, placebo-controlled trial. Graefes Arch Clin Exp Ophthalmol. 2006;244(5):551-8. 18. Saxena R, Singh D, Sharma M, et al. Steroids versus no steroids in nonarteritic anterior ischemic optic neuropathy: a randomized controlled trial. Ophthalmology. 2018 Apr 25. pii: S0161-6420(17)32715-X. 19. Pakravan M, Sanjari N, Sfandiari H, et al. The effect of high-dose steroids, and normobaric oxygen therapy, on recent onset non-arteritic anterior ischemic optic neuropathy: a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):2043-8. 20. Rebolleda G, Pérez-López M, Casas-LLera P, et al. Visual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroids. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):255-60. |

