 |
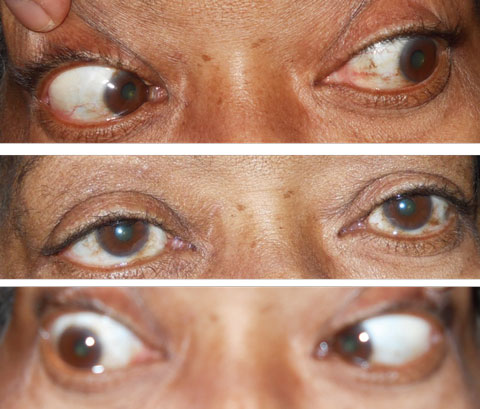 |
| Fig. 1. Note the normal movement of the eyes gazing to the left and straight in the top two photos. The bottom photo shows the patient’s eyes in right gaze and her inability to fully abduct the right eye. Click image to enlarge. |
The Patient
A 63-year-old woman presented to our office for examination with a chief complaint of new-onset diplopia starting approximately two months earlier. She described it as “side-by-side images” and even occasional “triple vision.” She also reported an increase in headaches for and that her overall vision had decreased. Also of note, the patient was established with a neurologist and was last seen two months earlier, at which point magnetic resonance imaging (MRI) was done that the patient self reports was normal.The patient had no prior history of strabismus or any ocular surgery, but did have a history of lung cancer in 2005 and relapse in 2014. When asked in clinic if her double vision improves with one eye covered, the patient stated that it goes away when her right eye is covered.
A former smoker, she had quit approximately 12 years earlier. She also had hypertension and hyperlipidemia, which she was controlling medically. She was not diabetic or borderline diabetic. Best-corrected visual acuity was 20/20 OU through her current glasses. Red desaturation and color vision were normal and no afferent pupillary defect was noted at the exam. Versions and ductions reveal a complete loss of abduction in the right eye and full range of motion in the left (Figure 1). In primary gaze, prism measurement showed a 40PD esotropia, OD. External examination was normal in each eye, and dilated fundus exam revealed no abnormalities.
How We Handled It
We performed a forced duction test on the right eye, which was negative. The patient’s blood pressure was checked in office and found to be 140/80 using her right arm.Based on her presentation, we determined it was most likely a VI CN palsy. The most common cause of VI CN palsy is ischemia due to either diabetes or hypertension. The next most common are tumor or increased intracranial pressure. If the medical history supports uncontrolled hypertension or diabetes, imaging at the initial presentation may not be necessary. In this case, the medical history does not support an ischemic cause, so we recommended imaging. That same day, the patient underwent MRI and magnetic resonance venography (MRV) of the head and orbit with and without contrast.
Blood work including erythrocyte sedimentation rate, C-reactive protein, and a complete blood count with A1c was also ordered.
The results of her blood work were normal, but the MRI and MRV both had significant findings. Compared with her MRI two months prior, a sizable (2.1x1.6x2.4cm) peri-sphenoid lesion abutting the right cavernous sinus and involving right Meckel’s cave was detected (Figure 2).
Additionally, two smaller enhancing brain nodules were found in the left parafalcine occipital lobe and in the left frontal lobe. All were considered suspicious for lung cancer metastases.
Follow-up
With the cause identified, it was time for our patient’s neurologist and neurosurgeon to take charge in treatment. But our roles as optometrists were not over. Working closely with neurology during treatment is vital to showing improvement in the lesion and its effects.The patient underwent five rounds of radiation and came in every four weeks for versions/duction testing and visual fields. While all cases may not need to be seen this frequently, all CN palsies should be monitored for improvement.
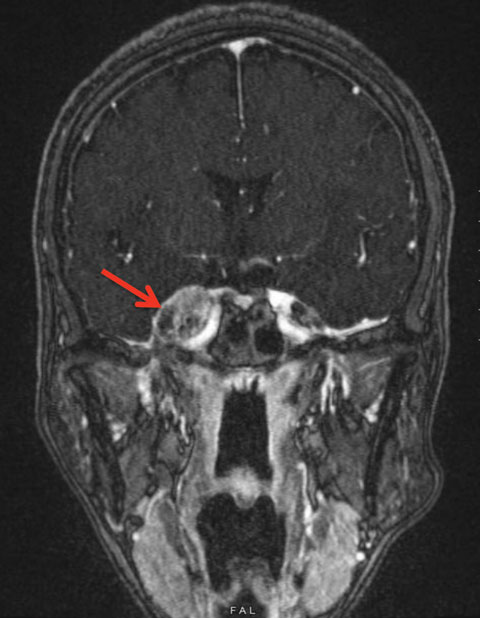 |
| Fig. 2. This is our patient’s FLAIR MRI with contrast of the head. The red arrow is pointing to the 2.1x1.6x2.4cm peri-sphenoid lesion abutting the right cavernous sinus and involving right Meckel’s cave. Click image to enlarge. |
Anatomy
Cranial nerves III, IV and VI control our extraocular muscles and each plays a specific role in the movement of our eyes. CN IV controls our superior oblique muscles, which control intorsion, depression and abduction.2,3 Loss of this muscle’s function causes an upward deviation of the affected eye with a cyclotorsion that causes the patient to tilt their head away from the lesion.2,3 This is the most common cause of acquired vertical diplopia that is worse on downgaze.4 CN VI controls our lateral rectus muscle, which controls abduction.5 With loss of innervation to this muscle, we are unable to turn the eye away from the midline and patients will often turn their head to avoid double vision.This leaves CN III to control all the other extraocular muscles and, when affected, tends to be the most dramatic, leaving the eye in a “down and out” position.5 CN III also controls the innervation of the levator muscle, which, if paralyzed, may also result in ptosis.5 The parasympathetic pupillary constricting fibers travel along the external portion of the CN III, which may be affected during a compressive lesion or aneurysm.3 An APD can be a sign of an aneurysm, which is emergent.3
When any one of these three cranial nerves is palsied it can result in diplopia, and the nerve experiencing the palsy should be identified.2-4,6 With CN III palsy, it is extremely important to monitor for pupillary involvement at the time of the exam and in the coming weeks thereafter.
Causes
Studies show that the most prevalent ocular CN palsy is that of CN VI, followed by CN III and then CN IV.7-11 The most common cause of acquired palsy in all three is ischemic changes from vascular diseases including diabetes, hypertension and atherosclerosis.2,12Mass lesions both in the orbit and in the brain are likely causes as well for CN III, IV and VI palsies. Depending on the location, a lesion or aneurysm on CN III can cause pupillary involvement.
Trauma is the third most common cause of these ocular palsies, with a higher occurrence of CN IV palsies related to the long distance it covers inside the cranial vault.5,9-11
Although not common, research shows a CN VI palsy can occur with giant cell arteritis (GCA).13
Treatment
In CN palsy cases involving the III, IV or VI nerve where the likely cause is ischemia, the patient should be monitored for improvement approximately a month after onset to make sure it is resolving. It may take three to six months before it is completely resolved. However, if a patient is appropriately making changes in blood sugar or blood pressure with their primary care doctor and not showing improvement in muscle movement recovery, it may indicate another pathology and warrant imaging. If the cause is indeterminate at the time of diagnosis, or a CN III palsy presents with an afferent pupillary defect, an MRI and MRV of head and orbit with and without contrast should be ordered. Be sure to order blood work to rule out undiagnosed hypercholesteremia or diabetes. In patients older than 50 years who have a CN VI and GCA is suspect, blood work including erythrocyte sedimentation rate and CRP must be ordered on a STAT basis.
Working hard to determine a source of diplopia in your patient caused by a CN palsy is more than just good care. It can be lifesaving.
Dr. Koetting practices at Virginia Eye Consultants, where she leads the externship program.
| 1. Gerstenblith A, Rainowitz M. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 6th ed. Philadelphia: Lippincott Williams and Wilkins;2012:2-3. 2. Miller N, Walsh F, Hoyt W. Walsh and Hoyt’s Clinical Neuro-ophthalmology, 6th edition. Philadelphia: Lippincott Williams & Wilkins. 2008. 3. Leigh J, Zee DS. The Neurology of Eye Movements. New York: Oxford University Press;2015. 4. Marais W, Barrett S. An overview of the third, fourth and sixth cranial nerve palsies. Continuing Medical Education [Online]. 2013;31(4):147-152. 5. Remington LA. Clinical anatomy of the visual system, 3rd edition. Philadelphia: Elsevier Health Science. 2011. 190-4. 6. Miller N, Newman N. Walsh and Hoyt’s Clinical neuro-ophthalmology 5th edition. Baltimore: Williams & Wilkins;1998:1194-223. 7. Park U, Kim S, Hwang J, Yu Y. Clinical features and natural history of acquired third, fourth and sixth cranial nerve palsy. Eye. 2008:22(5)691-6. 8. Rucker CW. Paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1958;46:787–94. 9. Rush J, Younge B. Paralysis of cranial nerves III, IV and VI. Cause and prognosis in 1,000 cases. Arch Ophthalmol. 1981;99:76–9. 10. Rucker C. The causes of paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1966;61:1293–8. 11. Rowe F, and VIS group UK Departments of Orthoptics, Multicentre UK recruiting centers, UK. Prevalence of ocular motor cranial nerve palsy and associations following stroke. Eye. 2011;25(7):881–7. 12. Park U, Kim S, Hwang J, Yu Y. Clinical features and natural history of acquired third, fourth, and sixth cranial nerve palsy. Eye (Lond). 2008 May;22(5):691-6. 13. Wilson CM, D’Ath P. A case of sixth cranial nerve palsy and suspected giant cell arteritis. BAOJ Ophthalmology. 2017;2(1):7. |

