| |
Volume 12, Number 1 |
March
2016 |
|
|
Inside
This Issue |
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
In December, we had our annual ORS meeting, Retina Update 2015, in conjunction with Review of Optometry. Over 100 ODs attended the meeting, held in Anaheim CA. There was a wide array of topics and lectures, but for me the real standouts were Stuart Richter’s keynote lecture on Epigenetics, and the lectures provided by our guest speaker, Retinal Specialist Hajir Dadgostar from the Retina Partners in the Greater Los Angeles area. By far my favorite was the panel discussion between myself, Mohammad Rafieetary, and Dr. Dadgostar, where we were able to pick his brains and really see how best to co-manage our pates with vitreoretinal disease. Thanks Mo and Hajir for a great lecture, and making me look smart!
LIVE
POLL
|
What do you think is a better treatment choice for your patients with PDR?
|
|
I also want to thank our sponsors: Centervue, Optovue, Second Sight and Zeavision. Optovue, a leader in OCT technology, and Zeavision, a nutraceutical company specializing in eye health, may already be familiar to many, and have been long time supporters of the ORS. Their continued support is crucial to our mission, and a mere thanks is probably not sufficient for all they have done, but it will have to do for now. The other companies may not be as familiar. Second Sight is the maker of the Argus II Retinal prosthesis, which has been implanted in over 30 patients with end stage RP. Centervue is a leader in the eye care diagnostics field, with several products already available, such as the DRS retinal camera and the Eidon , the first true color confocal laser, as well as new products on the way.
I also want to thank the staff of Review of Optometry, particularly Jonathan Dardine and Casey Foster. Without their help, we simply could not hold such a great meeting. Lastly, I want to thanks all the doctors who came to our meeting. I hope you found it as enjoyable as I did! And we hope to see you again in 2016, as we have started to plan for a meeting, with details to come.
Steven Ferrucci, O.D., F.A.A.O.
Editor in Chief
 |
PRESIDENT'S MESSAGE
I have been fortunate enough to be involved with utilizing injections throughout various stages of my practice career. When I first began practicing fresh out of school in 1993, I was doing an ocular disease residency at Omni Eye Services in Memphis, TN. At that time, optometrists in Tennessee had just gained the authority to perform diagnostic and therapeutic injections. To gain that authority, OD’s had to undergo a preceptorship with an Ophthalmologist and provide a certain number of supervised injections, which I did. Later, injectable certification could also be gained by taking an injection course that was provided by the Southern College of Optometry. For the six years that I practiced at Omni, I performed hundreds of injections consisting of intravenous injections for fluorescein angiography, intralesional injections of steroids into chalazia, and subconjunctival injections of steroids for chronic inflammation or CME. When we moved back to Indiana in 1999, injections by OD’s were prohibited here. I missed being able to perform them clinically, but did stay active in the area by providing the injection training for all of our students here at the IU School of Optometry. After several battles, Indiana OD’s finally gained the ability to perform injections about two years ago, becoming one of 14 states where OD’s can perform at least some degree of therapeutic or diagnostic injections. In 22 more states, OD’s can perform only intramuscular injections for the purpose of counteracting anaphylaxis. I must admit, that I have really enjoyed being able to clinically help my patients once again with injections when needed, and I continue to enjoy teaching injection skills to our students.
NOVEMBER 2015
POLL RESULTS
|
So what does all of this have to do with the retina? Now, you’ll notice that I did not mention intravitreal injections above when discussing the injections that we perform. At this time, OD’s in the United States are typically not involved with these injections, and many states with injectable authority for OD’s specifically prohibit them. Could we be performing them? Should we be? In 2014, the results of a very interesting study were published in Eye, 28(6), pages 734-740. Retinal specialists in England trained nurse practitioners to give intravitreal injections. Yes, nurse practitioners. Out of 4000 injections provided in this manner, the only complication encountered was subconjunctival hemorrhage, and this occurred in 5.7% of the patients. So in England, retinal surgeons found it effective, safe, and efficient to train nurse practitioners to give intravitreal injections. At the very least, that is certainly something to ponder.
Brad Sutton, O.D., F.A.A.O.
ORS President

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

CLINICAL
PEARLS
The Argus II Retinal Prosthesis
By: Steven Ferrucci, OD, FAAO
ORS Fellow
The Argus II Retinal Prosthetic System (Second Sight Medical Products, Inc., , Sylmar, CA), was approved February 2013, by the US Food and Drug Administration. The approval is currently limited to patients who have lost sight from retinitis pigmentosa (RP), and are currently NLP or bare LP. The implant allows some patients with profound RP to locate objects, detect movement, and improve orientation and mobility skills.
The unit consists of a surgically implanted 60-electorde array over the macula, an inductive coil link used to transmit power and video data to the implant, an external belt-worn video processing unit (VPU), and a miniature video camera mounted on a pair of glasses. The video camera captures visual information, and relays the information to the VPU. The VPU in turn digitizes the signal in real time, and creates a series of stimulus pulses. These pulses are then transmitted to the microelectrode array on the retina, where they stimulate the remaining cells in the retina. The nerve cells in the retina transmit the visual information along the optic nerve to the brain, creating the perception of patterns of light. The patient then must learn to interpret these visual patterns, regaining some functional vision.
While only implanted in a relatively small number of patients, the Argus II represents a significant step forward in returning some function to eyes with devastating visual loss for RP. On-going research and advances will no doubt create and even better system, hopefully helping even more patients in the future with vision loss from other diseases as well.
EIDON Wide Field True Color Confocal Scanner
By Steven Ferrucci, OD, FAAO
ORS Fellow
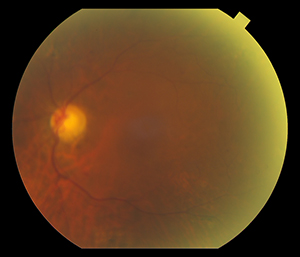
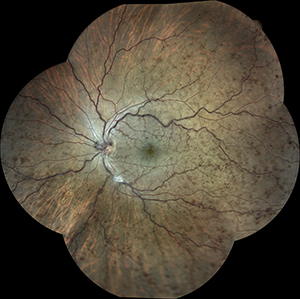
I have been using the Eidon Wide-Field, True Color Confocal Color Scanner in my clinic. The images that we have taken are excellent, far superior in clarity and detail to my standard retinal camera. Further, the combination white light illumination and cong=focal imaging enables penetration through cataracts and other media opacity while still obtaining great images. My favorite feature is the mosaic, which allows you to merge photos from 6 separate fields in one wide-field photo, reaching 90 by 110 degrees.
Almost as good as the quality is the ease of use. The system is completely automated, and is able to locate the patient’s eye and automatically focus, obtaining clear pictures every time. It reduces the technical skill needed to obtain images, and is much easier to train staff on than a traditional camera.
 |
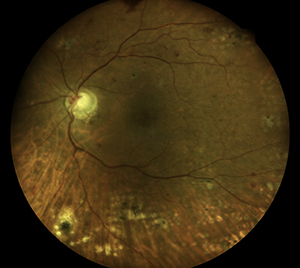 |
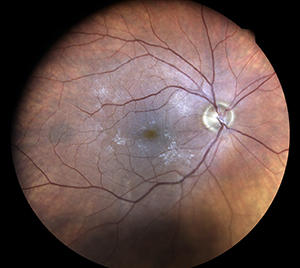
Photo taken with standard retina camera (left) and Eidon (right) in a diabetic pt with 2 + nuclear sclerotic cataracts. Note improved detail with Eidon.
|
 |
 |
6 field Mosaic of a Patient with
CRVO OS taken with the Eidon |
ERM OD taken with the Eidon |
|

JOURNAL
ABSTRACTS
Long-term Follow-up and Outcomes in Traumatic Macular Holes
The purpose of this retrospective case series was to review presenting characteristics, clinical course, and long-term visual and anatomic outcomes of twenty-eight consecutive patients with traumatic macular holes at a single tertiary referral center.
In addition to visual acuities and treatments throughout the clinical course, specific dimensions of the macular hole, including diameters, height, configuration, shape, and the presence of a cuff of fluid, were examined using spectral-domain optical coherence tomography (OCT).
Twenty-eight patients were identified with a mean initial visual acuity (VA) of logMAR 1.3 (20/400) and a mean follow-up of 2.2 years. Eleven holes (39.3%) closed spontaneously in median 5.7 weeks. Eleven underwent vitrectomy with a median time to intervention of 35.1 weeks. Median time to surgery for the 5 eyes with successful hole closure was 11.0 weeks vs 56.3 weeks for the 6 eyes that failed to close (P = .02). VA improved in closed holes (P < .01), whether spontaneously (P < .01) or via vitrectomy (P = .04), but VA did not improve in holes that did not close (P = .22). There was no relation between initial OCT dimensions and final hole closure status, although there was a trend, which did not reach statistical significance, toward small dimensions for those that closed spontaneously.
In conclusion, the study observed a fairly high spontaneous closure rate of traumatic macular holes, with a trend toward smaller OCT dimensions. However no relationship was found between hole closure and the OCT characteristics of the hole. Surgical intervention was less successful at hole closure when elected after 3 months.
Miller JB, Yonekawa Y, Eliott D, et al. Am J Ophthalmol. 2015 Dec;160(6):1255-1258
Gene Therapy for Leber Hereditary Optic Neuropathy
Leber hereditary optic neuropathy (LHON) is a disorder characterized by severe and rapidly progressive visual loss when caused by a mutation in the mitochondrial gene encoding NADH:ubiquinone oxidoreductase subunit 4 (ND4). We have initiated a gene therapy trial to determine the safety and tolerability of escalated doses of an adeno-associated virus vector (AAV) expressing a normal ND4 complementary DNA in patients with a G to A mutation at nucleotide 11778 of the mitochondrial genome.
In this prospective open-label trial (NCT02161380), the study drug (self-complementary AAV [scAAV]2(Y444,500,730F)-P1ND4v2) was intravitreally injected unilaterally into the eyes of 5 blind participants with G11778A LHON. Four participants with visual loss for more than 12 months were treated. The fifth participant had visual loss for less than 12 months. The first 3 participants were treated at the low dose of vector (5 × 109 vg), and the fourth participant was treated at the medium dose (2.46 × 1010 vg). The fifth participant with visual loss for less than 12 months received the low dose. Treated participants were followed for 90 to 180 days and underwent ocular and systemic safety assessments along with visual structure and function examinations.
Visual acuity as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) eye chart remained unchanged from baseline to 3 months in the first 3 participants. For 2 participants with 90-day follow-up, acuity increased from hand movements to 7 letters in 1 and by 15 letters in 1, representing an improvement equivalent to 3 lines. No one lost vision, and no serious adverse events were observed.
No serious safety problems were observed in the first 5 participants enrolled in this phase I trial of virus-based gene transfer in this mitochondrial disorder. Additional study follow-up of these and additional participants planned for the next 4 years is needed to confirm these preliminary observations.
Feuer WJ, Schiffman JC, Davis JL, et al. Ophthalmology, Volume 123, Issue 3, Pages 558–570.
Rates of Vitrectomy among Enrollees in a United States Managed Care Network, 2001–2012
The purpose of this study was to determine whether vitrectomy surgery rates have changed over the past decade and factors affecting the odds of undergoing this procedure. This was a retrospective, longitudinal cohort study of patients 21 years of age or older between 2001 and 2012 in a United States managed care network.
Claims data from a managed care network were analyzed to identify all enrollees who underwent 1 vitrectomy or more each year from 2001 through 2012. Rates of vitrectomy per 1000 enrollees were computed each year from 2001 through 2012 for the entire group and separately for patients with and without diabetes mellitus. Multivariate logistic regression assessed factors affecting the odds of undergoing vitrectomy surgery.
Among the 11,161,907 eligible enrollees, 40 892 (0.4%) underwent vitrectomy over the 12-year period. The average age of those undergoing vitrectomy was 57±13 years. Overall vitrectomy rates increased 31% from 2001 to 2012 (from 1.47 to 1.92 per 1000 patients). During this same period, the vitrectomy rate among persons with diabetes mellitus decreased by 43% (from 5.84 to 3.31 per 1000 patients with diabetes). Women had 24% decreased odds of undergoing vitrectomy (adjusted odds ratio [OR], 0.76; 95% confidence interval [CI], 0.72–0.79). The odds of undergoing a vitrectomy were 17% greater for black persons (adjusted OR, 1.17; 95% CI, 1.07–1.27) and 7% higher for persons with diabetes (adjusted OR, 1.07; 95% CI, 1.01–1.14).
Overall, there was an increase in the vitrectomy rates per 1000 enrollees in this large managed care network over the course of the past decade. However, among persons with diabetes mellitus, vitrectomy rates declined substantially over this period. These changes may be explained, in part, by advances in surgical instrumentation and imaging methods to detect retinal diseases changing among other.
Wubben TJ, Talway N, Blachley TS, et al. Ophthalmology March 2016, Volume 123, Issue 3, Pages 590–598.
Outcomes in Eyes with Retinal Angiomatous Proliferation in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT)
The purpose of this study was to compare baseline characteristics, visual acuity (VA), and morphologic outcomes between eyes with retinal angiomatous proliferation (RAP) and all other eyes among patients with neovascular age-related macular degeneration (NVAMD) treated with anti–vascular endothelial growth factor (VEGF) drugs from the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT).
Reading center staff evaluated digital color fundus photographs, fluorescein angiography (FA) images, and optical coherence tomography (OCT) scans of eyes with NVAMD treated with either ranibizumab or bevacizumab over a 2-year period. Retinal angiomatous proliferation was identified by the intense intra-retinal leakage of fluorescein in combination with other associated features.
Retinal angiomatous proliferation was present in 126 of 1183 (10.7%) study eyes at baseline. Mean VA improvement from baseline was greater (10.6 vs. 6.9 letters; P = 0.01) at 1 year, but similar at 2 years (7.8 vs. 6.2 letters; P = 0.34). At 1 year, eyes with RAP were more likely to have no fluid (46% vs. 26%; P < 0.001) on OCT, no leakage on FA (61% vs. 50%; P = 0.03), and greater reduction in foveal thickness (−240 μm vs. −161 μm; P < 0.001). They were more likely to demonstrate GA (24% vs. 15%; P = 0.01) and less likely to have scarring (17% vs. 36%; P < 0.001) or SHRM (36% vs. 48%; P = 0.01). These results were similar at 2 years. The mean change in lesion size at 1 year differed (−0.27 DA vs. 0.27 DA; P = 0.02), but was similar at 2 years (0.49 DA vs. 0.79 DA; P = 0.26). Among eyes treated PRN, eyes with RAP received a lower mean number of injections in year 1 (6.1 vs. 7.4; P = 0.003) and year 2 (5.4 vs. 6.6; P = 0.025).
At both 1 and 2 years after initiation of anti-VEGF treatment in CATT, eyes with RAP were less likely to have fluid, FA leakage, scar, and SHRM and more likely to have GA than eyes without RAP. Mean improvement in VA was similar at 2 years.
Daniel E, Shaffer J, Ying GS, et al for the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group∗. March 2016Volume 123, Issue 3, Pages 609–616.
Risk Factors and Incidence of Macular Edema after Cataract Surgery
A Database Study of 81984 Eyes
The purpose of this study was to define the incidence of pseudophakic macular edema (PME) after cataract surgery and to identify contributory risk factors. This was a retrospective database study of electronic medical records (EMRs). A total of 81,984 eyes undergoing cataract surgery between December 2010 and December 2014 from 8 independent United Kingdom clinical sites were evaluated. This included patients that had a diagnosis of cystoid macular edema or new-onset macular edema in patients with diabetes, recorded by a healthcare professional within 90 days of surgery.
Baseline incidence of PME in eyes without operative complications, diabetes, or risk factors was 1.17%. Eyes in which PME developed were more likely to be male, older, and to demonstrate risk factors. The relative risk (RR) was increased in eyes with capsule rupture with or without vitreous loss (RR, 2.61; 95% confidence interval [CI], 1.57–4.34), a previous diagnosis of epiretinal membrane (RR, 5.60; 95% CI, 3.45–9.07), uveitis (RR, 2.88; 95% CI, 1.50–5.51), retinal vein occlusion (RR, 4.47; 95% CI, 2.56–5.92), or retinal detachment repair (RR, 3.93; 95% CI, 2.60–5.92). High myopia, age-related macular degeneration, or prostaglandin analog use were not shown to increase risk. Eyes with PME on average had poorer postoperative visual acuity, which persisted to the latest time point assessed, up to 24 weeks. Eyes from patients with diabetes, even in the absence of retinopathy, had an increased RR (RR, 1.80; 95% CI, 1.36–2.36) of new macular edema after surgery. The risk was higher in the presence of any diabetic retinopathy (DR; RR, 6.23; 95% CI, 5.12–7.58) and rose proportionately with increasing severity of DR.
The study concluded that pseudophakic macular edema occurs commonly after phacoemulsification cataract surgery, even in the absence of complications and risk factors. This large retrospective study using structured EMR data quantified the RRs of PME and the risk with increasing ETDRS severity of DR. It highlights the need for prophylactic therapy, especially in those groups of eyes with the highest RRs.
Chu CJ, Johnston RL, Buscombe C, et al. Ophthalmology February 2016, Volume 123, Issue 2, Pages 316–323.
Rates of Reoperation and Retinal Detachment after Macular Hole Surgery
The purpose of this study was to evaluate rates of reoperation and retinal detachment (RD) after macular hole surgery. This was a Retrospective cross-sectional study of patients in the insurance claim–based MarketScan databases from 2007 through 2013 with a record of macular hole surgery.
Patients with macular hole surgery were identified and cases of definite (the same eye was coded both times) and presumed (the eye laterality was not coded) macular hole reoperations within 2, 3, and 12 months were queried. In addition, cases of postoperative RD within 2, 3, and 12 months were captured.
Records of 23,465 macular hole surgeries among 20,764 patients were analyzed. Among presumed reoperations, the rates of reoperation were 4.3% (4.1% after ILM peeling and 5.0% after no ILM peeling; P = 0.01) within 2 months of surgery, 5.5% (5.3% after ILM peeling and 6.2% after no ILM peeling; P = 0.03) within 3 months of surgery, and 9.5% (9.0% after ILM peeling and 11.0% after no ILM peeling; P = 0.01) within 12 months of surgery. The rates for definite reoperations were 1.3% (1.2% after ILM peeling and 1.8% after no ILM peeling; P = 0.04) at 2 months, 1.7% (1.6% after ILM peeling and 2.5% after no ILM peeling; P = 0.004) at 3 months, and 4.1% (3.3% after ILM peeling and 7.5% after no ILM peeling; P < 0.001) at 12 months. The cumulative rate of postoperative RD was 1.81±0.09% to 2.18±0.5% after 2 months, 2.27±0.10% to 3.18±0.67% after 3 months, and 3.92±0.16% to 5.70±1.1% after 12 months. Internal limiting membrane peeling was associated negatively with postoperative RD at 2 months (2.3% vs. 1.7%; P = 0.007), 3 months (2.8% vs. 2.1%; P = 0.004), and 12 months (4.7% vs. 3.3%; P < 0.001).
The authors concluded that reoperations for macular hole were performed at low rates. Internal limiting membrane peeling was associated with lower rates of reoperation and RD.
Vaziri K, Schwartz SG, Kishor K, et al. Ophthalmology January 2016, Volume 123, Issue 1, Pages 26–31.
Comparison of Prevalence of Diabetic Macular Edema Based on Monocular Fundus Photography vs Optical Coherence Tomography
The goal of this study was to compare DME prevalence from monocular fundus photography and OCT. Epidemiologic studies and telemedicine screening typically use monocular fundus photography, while treatment of DME uses OCT CST.
This was a retrospective cross-sectional study of DME grading based on monocular fundus photographs and OCT images obtained from patients with diabetic retinopathy at a single visit between July 1, 2011, and June 30, 2014, at a university-based practice and analyzed between July 30, 2014, and May 29, 2015. Presence of DME, including clinically significant macular edema (CSME), on monocular fundus photographs used definitions from the Multi-Ethnic Study of Atherosclerosis (MESA) and the National Health and Nutrition Examination Survey (NHANES). Presence of DME on OCT used Diabetic Retinopathy Clinical Research Network eligibility criteria thresholds of CST for trials evaluating anti–vascular endothelial growth factor treatments.
A total of 246 eyes of 158 participants (mean [SD] age, 65.0 [11.9] years; 48.7% women; 60.8% white) were included. Among the 246 eyes, the prevalence’s of DME (61.4%) and CSME (48.5%) based on MESA definitions for monocular fundus photographs were greater than the DME prevalence based on OCT (21.1%) by 40.2% (95% CI, 32.8%-47.7%; P < .001) and 27.2% (95% CI, 19.2%-35.3%; P < .001), respectively. Using NHANES definitions, DME and CSME prevalences from monocular fundus photographs (28.5% and 21.0%, respectively) approximated the DME prevalence from OCT (21.1%). However, among eyes without DME on OCT, 58.2% (95% CI, 51.0%-65.3%) and 18.0% (95% CI, 12.9%-24.2%) were diagnosed as having DME on monocular fundus photographs using MESA and NHANES definitions, respectively, including 47.0% (95% CI, 39.7%-54.5%) and 10.3% (95% CI, 6.3%-15.7%), respectively, with CSME. Among eyes with DME on OCT, 26.9% (95% CI, 15.6%-41.0%) and 32.7% (95% CI, 20.3%-47.1%) were not diagnosed as having either DME or CSME on monocular fundus photographs using MESA and NHANES definitions, respectively.
These data from this study suggests that many eyes diagnosed as having DME or CSME on monocular fundus photographs have no DME based on OCT CST, while many eyes diagnosed as not having DME or CSME on monocular fundus photographs have DME on OCT.
Wang YT, Tadarati M, Wolfson Y, et al. JAMA Ophthalmol. 2016;134(2):222-228. doi:10.1001/jamaophthalmol.2015.5332.
Comparison of intravitreal bevacizumab and dexamethasone implant for the treatment of macula oedema associated with branch retinal vein occlusion
The goal of this study was to compare DME prevalence from monocular fundus photography and OCT. Epidemiologic studies and telemedicine screening typically use monocular fundus photography, while treatment of DME uses OCT CST.
This was a retrospective cross-sectional study of DME grading based on monocular fundus photographs and OCT images obtained from patients with diabetic retinopathy at a single visit between July 1, 2011, and June 30, 2014, at a university-based practice and analyzed between July 30, 2014, and May 29, 2015. Presence of DME, including clinically significant macular edema (CSME), on monocular fundus photographs used definitions from the Multi-Ethnic Study of Atherosclerosis (MESA) and the National Health and Nutrition Examination Survey (NHANES). Presence of DME on OCT used Diabetic Retinopathy Clinical Research Network eligibility criteria thresholds of CST for trials evaluating anti–vascular endothelial growth factor treatments.
A total of 246 eyes of 158 participants (mean [SD] age, 65.0 [11.9] years; 48.7% women; 60.8% white) were included. Among the 246 eyes, the prevalence’s of DME (61.4%) and CSME (48.5%) based on MESA definitions for monocular fundus photographs were greater than the DME prevalence based on OCT (21.1%) by 40.2% (95% CI, 32.8%-47.7%; P <.001) and 27.2% (95% CI, 19.2%-35.3%; P <.001), respectively. Using NHANES definitions, DME and CSME prevalences from monocular fundus photographs (28.5% and 21.0%, respectively) approximated the DME prevalence from OCT (21.1%). However, among eyes without DME on OCT, 58.2% (95% CI, 51.0%-65.3%) and 18.0% (95% CI, 12.9%-24.2%) were diagnosed as having DME on monocular fundus photographs using MESA and NHANES definitions, respectively, including 47.0% (95% CI, 39.7%-54.5%) and 10.3% (95% CI, 6.3%-15.7%), respectively, with CSME. Among eyes with DME on OCT, 26.9% (95% CI, 15.6%-41.0%) and 32.7% (95% CI, 20.3%-47.1%) were not diagnosed as having either DME or CSME on monocular fundus photographs using MESA and NHANES definitions, respectively.
These data from this study suggests that many eyes diagnosed as having DME or CSME on monocular fundus photographs have no DME based on OCT CST, while many eyes diagnosed as not having DME or CSME on monocular fundus photographs have DME on OCT.
Wang YT, Tadarati M, Wolfson Y, et al. JAMA Ophthalmol. 2016;134(2):222-228. doi:10.1001/jamaophthalmol.2015.5332.
Optical Coherence Tomography Angiography and En Face Optical Coherence Tomography Features of Paracentral Acute Middle Maculopathy
The purpose of this retrospective observational case series was to characterize the optical coherence tomography (OCT) angiography, en face OCT, and microperimetry features of paracentral acute middle maculopathy in both the acute phase and after resolution, and to propose a classification of distinct subtypes of this entity.
Clinical histories, high-resolution digital color imaging, spectral-domain OCT images, fluorescein angiography, OCT angiography images, and en face OCT images of 16 patients with paracentral acute middle maculopathy were evaluated. In addition, microperimetry was available in 6 patients.
The most common referring diagnoses were isolated branch retinal arterial occlusion (5/16), combined central retinal vein and cilioretinal artery occlusion (4/16), and isolated central retinal vein occlusion (4/16). All patients demonstrated hyperreflective plaque-like lesions at the level of the inner nuclear layer on spectral-domain OCT, with no fluorescein angiographic correlate. OCT angiography demonstrated variable areas of capillary dropout within the superficial and deep retinal capillary plexi in these areas. En face OCT highlighted confluent areas of middle retina hyperreflectivity corresponding to these lesions. Three distinct en face OCT patterns were observed: arteriolar, fern-like, and globular. Microperimetry demonstrated relative scotomas mapping to the area of middle retinal hyperreflectivity seen on en face OCT.
Paracentral acute middle maculopathy may be best evaluated with the use of en face OCT imaging, which corresponds to subjective and objective visual field defects. En face OCT appearance may be used to classify paracentral acute maculopathy into distinct subtypes.
Sridhar J, Shahlaee A, Rahimy E, et al. Am J Ophthalmol. 2015 Dec;160(6):1259-1268.
Peripheral Cryoablation for Treatment of Active Pars Planitis: Long-Term Outcomes of a Retrospective Study
The purpose of this retrospective comparative case series was to investigate the outcome of management of traditional therapy vs cryoablation for active pars planitis.
136 of 186 eyes studied were treated conventionally (with topical, locally injected, or oral corticosteroid therapy), and 50 eyes were treated with cryotherapy.
Two rows of cryotherapy were applied in areas of snowbanking along the areas of involved pars plana. Cryotherapy was later repeated if inflammation remained active. Sub-Tenon kenalog or oral steroids with a rapid taper were used adjunctively at the time of the procedure period to blunt any immediate inflammation.
In cases of traditional corticosteroid treatment, patients received either topical prednisolone acetate 1% drops, intravitreal triamcinolone acetonide injection, sub-Tenon triamcinolone acetonide injection, or oral prednisone.
In measures of both vitreous and anterior chamber cell, cryotherapy-treated eyes were shown to have a higher chance of resolution at one year and six month follow-ups, respectively. Cryotherapy appeared to have higher rates of CME resolution at six months and one year. Over the course of the retrospective review, VA had a trend toward slightly worsening vision in the traditionally-managed patient cohort and a trend toward slightly improving vision in the cryotherapy-treated patient cohort.
Peripheral cryotherapy as a first-line treatment in active cases of pars planitis may prove to have better immediate outcomes than traditional therapy with corticosteroid treatment.
Sohn E, Chaon B, Jabs D, Folk J. American Journal of Ophthalmology. February 2016. 162: 35-42.
Natural History of Geographic Atrophy Progression Secondary to Age-Related Macular Degeneration (Geographic Atrophy Progression Study)
The Geographic Atrophy Progression (GAP) study was designed to assess the rate of geographic atrophy (GA) progression and to identify prognostic factors by measuring the enlargement of the atrophic lesions using fundus autofluorescence (FAF) and color fundus photography (CFP).
This was a prospective, multicenter, noninterventional natural history study involving a total of 603 participants. 413 of those had gradable lesion data from FAF or CFP, and 321 had gradable lesion data from both FAF and CFP.
Atrophic lesion areas were measured by FAF and CFP to assess lesion progression over time. Lesion size assessments and best-corrected visual acuity (BCVA) were conducted at screening/baseline (day 0) and at 3 follow-up visits: month 6, month 12, and month 18 (or early exit).
Mean (standard error) lesion size changes from baseline, determined by FAF and CFP, respectively, were 0.88 (0.1) and 0.78 (0.1) mm2 at 6 months, 1.85 (0.1) and 1.57 (0.1) mm2 at 12 months, and 3.14 (0.4) and 3.17 (0.5) mm2 at 18 months. The mean change in lesion size from baseline to month 12 was significantly greater in participants who had eyes with multifocal atrophic spots compared with those with unifocal spots (P < 0.001) and those with extrafoveal lesions compared with those with foveal lesions (P = 0.001). The mean (standard deviation) decrease in visual acuity was 6.2 ± 15.6 letters for patients with image data available. Atrophic lesions with a diffuse (mean 0.95 mm2) or banded (mean 1.01 mm2) FAF pattern grew more rapidly by month 6 compared with those with the “none” (mean, 0.13 mm2) and focal (mean, 0.36 mm2) FAF patterns.
The authors concluded that although differences were observed in mean lesion size measurements using FAF imaging compared with CFP, the measurements were highly correlated with one another. Significant differences were found in lesion progression rates in participants stratified by hyperfluorescence pattern subtype. This large GA natural history study provides a strong foundation for future clinical trials.
Schmitz-Valckenberg, S, Sahel JA, Danis R, et al. Ophthalmology, February 2016, Volume 123, Issue 2, Pages 361–368

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
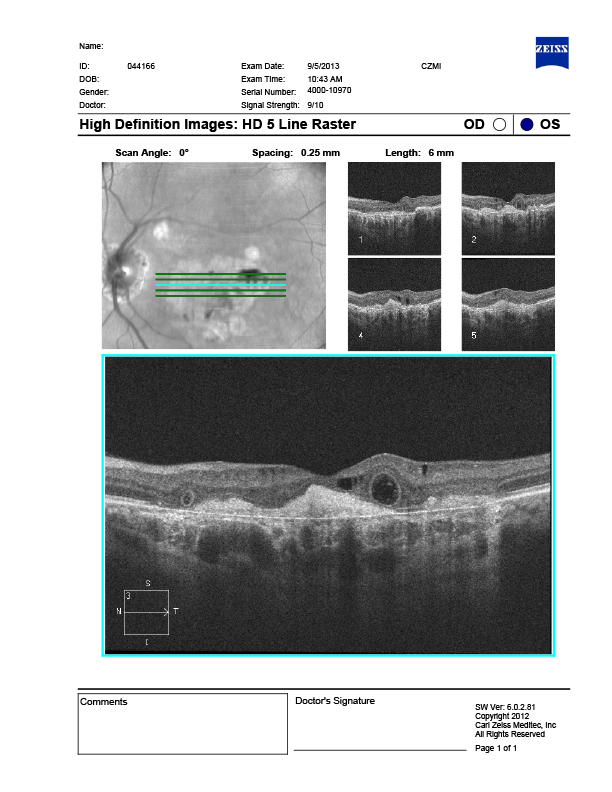
This OCT represents outer retinal tubulations (ORTs). The advent of SD-OCT has led to superb imaging capabilities in addition to enhanced visualization of the retinal layers. Such advancements have led to the identification of a variety of new retinal conditions, including outer retinal tubulations (ORTs). ORTs are ovoid hyporeflective spaces located in the outer retina. The pathogenesis is unclear but appears to involve sub-lethal injury to the photoreceptors leading to a compensatory reorganization of the photoreceptor layer with the neighboring ellipsoid zone resulting in a hyper-reflective border surrounding a central lumen. Most ORTs have been linked to wet AMD, however, these peculiar structures are now seen in a myriad of retinal disorders.
Recognition of ORT is clinically relevant, as these tubules are a morphologic anomaly and do not appear to represent active leakage and can only be denoted through the use of SD-OCT. Although ORTs were once linked exclusively to Wet AMD, it is now recognized in a number of macular conditions that contribute to disruption of the outer retina, photoreceptors and RPE. The understanding of ORT may help provide a better understanding of the natural progression of several macular conditions, thus aiding in their management.
Julie Rodman, OD, FAAO, ORS Fellow
Brad Sutton, OD, FAAO, ORS Fellow
Diana Shechtman, OD, FAAO ORS Fellow
|

IN THE
NEWS
 |
AngioVue imaging system receives clearance from FDA
The FDA has cleared the AngioVue imaging system, Optovue announced in a press release by the company.
The device is now commercially available in the U.S.
AngioVue provides physicians with a noninvasive method of visualizing abnormal blood cells in the retina to help determine an appropriate course of treatment.
“We are thrilled to announce FDA clearance for our AngioVue system, which will bring significant benefits of our innovative, noninvasive retinal imaging to patients in the U.S. suffering from retinal diseases that lead to progressive blindness,” Jay Wei, founder and CEO of Optovue, said in the release
|
 |
Allegro Ophthalmics Announces Last Patient Enrolled in Del Mar Phase 2b Clinical Trial of Luminate for the Treatment of DME
Allegro Ophthalmics, LLC, a biotechnology company focused on the development of therapies to treat vitreoretinal diseases, today announced completion of enrollment in its DEL MAR Phase 2b trial that is evaluating the safety and efficacy of Luminate® (ALG-1001) in patients with diabetic macular edema (DME). The company expects to report top-line data by third quarter 2016.
“Completing the last patient enrollment in the DEL MAR trial on schedule represents another significant clinical development milestone for this drug candidate, and moves us a step closer to potentially bringing this new category of treatment forward to help DME patients,” says Vicken Karageozian, MD, Chief Technical Officer, Allegro Ophthalmics, LLC. “The protocol follows the prospective analysis of the Phase 1 DME study, which demonstrated strong safety and efficacy in monotherapy treatment with Luminate. We are cautiously optimistic that Phase 2b data will show similar outcomes.”
“I’m pleased to be participating in this important study which has the potential to benefit the millions of diabetics who are at risk of losing their vision,” says David Boyer, MD, Clinical Professor of Ophthalmology at USC/Keck School of Medicine and member of Allegro’s Scientific Advisory Board. “There is a significant need to find better treatment options that reduce the number of intravitreal injections, while improving vision for our diabetic patients. In clinical studies to date, Luminate has been shown to be an enhanced clinical benefit for those patients who either don’t respond to anti-VEGF therapy or plateau with less than excellent vision.”
|
 |
Regeneron reports 46% increase in total revenue for year
Regeneron reported a year-end total revenue of $4.1 billion for 2015 compared with $2.8 billion in 2014, according to a company press release.
The 46% increase was partially attributed to an increase in the company’s net profit from commercialization of Eylea (aflibercept, Regeneron) outside of the United States.
Net product sales of Eylea in the U.S. were reported at $2.7 billion for the full year compared with $1.7 billion in 2014.
Non-GAAP income was reported at $1.4 billion, or $13.52 per basic share and $12.07 per diluted share, for the full year compared with $1.18 billion, or $11.68 per basic share and $10.00 per diluted share, in 2014.
|
 |
Patients with macular degeneration show improvement with high-dose statin treatment
Researchers found evidence that treatment with high-dose atorvastatin (80mg) is associated with regression of lipid deposits and improvement in visual acuity, without progression to advanced disease, in high-risk AMD patients. Their findings were published in EBioMedicine--a new online journal led by editors of the journals Cell and The Lancet--and not only further the connection between lipids, AMD and atherosclerosis, but also present a potential therapy for some patients with dry AMD.
Ophthalmologists and vision researchers have long suspected that there may be a connection between dry AMD and atherosclerosis. In dry AMD, physicians often see soft, lipid-rich drusen in the outer retina, similar to the build-up of lipid material in the inner walls of blood vessels in atherosclerosis. Statin use is widespread in middle-aged and older individuals, who also have an increased risk of AMD; however, previous studies have shown very little correlation between regular statin use and improvements in AMD. The authors of the EBioMedicine paper hypothesized that, due to the heterogeneous nature of the disease, patients with soft, lipid-rich drusen may respond better to statins prescribed at higher dosages.
Twenty-three patients with dry AMD marked by soft lipid deposits in the outer retina were prescribed a high dose (80mg) of atorvastatin, the generic name of the statin marketed as Lipitor® and several generic equivalents. Of the 23 patients, 10 experienced an elimination of the deposits under the retina and mild improvement in visual acuity. Other techniques that have attempted to eliminate the deposits have mostly failed with the disease continuing to progress to more advanced dry AMD or a conversion to the wet form of AMD.
As the next step for this line of research, the investigators plan to expand to a larger prospective multicenter trial to further investigate the efficacy of the treatment in a larger sample of patients with dry AMD.
|
 |
VA, morphologic features strengthen during year 2 of CATT
The associations between visual acuity and morphologic features on fundus photography, fluorescein angiography and optical coherence tomography in the second year of the Comparison of Age-related Macular Degeneration Treatment Trials were maintained or strengthened during year 2, according to a study in Ophthalmology.
CATT Study participants required angiographic and optical coherence tomography evidence of choroidal neovascularization (CNV) secondary to age-related macular degeneration and visual acuity between 20/25 and 20/320. Participants were randomly assigned to receive ranibizumab or bevacizumab with three different dosing regimens over a 2-year period.
Sharma and colleagues reported that anti-VEGF treatment did not further reduce macular fluid, thickness or vascular leakage, or stabilize lesion growth, which contrasted the decreases in year 1 of the study. Visual acuity gains were maintained or improved by anti-VEGF therapy for all groups except the ranibizumab switched group (patients who were randomly selected to switch to an as-needed dosing regimen).
The largest decreases in visual acuity were associated with intraretinal fluid, abnormally thin or thick retinas, larger CNV area, increasing subretinal pigment epithelium tissue complex thickness, and foveal scar and geographic atrophy at week 104, according to the study.
A key 1-year finding, that intraretinal fluid, as determined by OCT, had a negative impact on visual acuity at all time points examined, was strengthened throughout year 2.
Researchers determined that the presence of subretinal fluid was associated with better visual acuity at year 2 even when controlling for other potentially confounding variables.
Worse visual acuity was seen in abnormally thin or thick retinas, which was strengthened over year 2.
|
 |
Study shows possible genetic link to persistent retinal fluid in AMD
Persistent retinal fluid in eyes that have been treated with anti-VEGFs for neovascular age-related macular degeneration may have a genetic basis, Phil Rosenfeld, MD, colleagues at the Angiogenesis, Exudation, and Degeneration Meeting in Miami. Rosenfeld and colleagues culled data from the VIEW 1 trial, which evaluated the efficacy and safety of Eylea (aflibercept, Regeneron) compared with Lucentis (ranibizumab, Genentech) in the treatment of neovascular AMD.
Rosenfeld and colleagues analyzed blood samples from 323 patients, conducted a genome-wide association study and coded genetic variants for each single nucleotide polymorphism (SNP).
SNPs on the X chromosome showed the highest suggestive genetic association with the presence of retinal fluid at 52 weeks, Rosenfeld said. More specifically, Rosenfeld and colleagues identified a novel genetic variant in the protein kinase X-linked (PRKX) gene, which is involved in angiogenesis through stimulation of endothelial cell proliferation, migration and vascular-like structure formation. The novel genetic variant in the PRKX gene was associated with a reduced likelihood of macular fluid being present at week 52 in the VIEW 1 trial.
|
 |
Protein targets tissue factor, angiogenesis in CNV related to AMD
A protein that targets tissue factor in neovascular age-related macular degeneration was well tolerated in a phase 1 study, a Carl D. Regillo, MD told colleagues at the Angiogenesis, Exudation, and Degeneration 2016 meeting in Miami, while reviewing results of a phase 1 study of ICON-1 (Iconic Therapeutics) in the treatment of neovascular AMD.
ICON-1 is a human immunoconjugate fusion protein that targets tissue factor. In pathology, tissue factor overexpression triggers angiogenesis, tumor growth and metastasis, and promotes an inflammatory immune environment via extra- and intracellular signaling. Tissue factor is involved in fibrin formation and growth of the CNV complex, Regillo said.
The study included 18 patients with active choroidal neovascularization related to AMD. Six patients received a single 60-µg injection of ICON-1, six patients received a single 150-µg injection of ICON-1, and six patients received a single 300-µg injection of ICON-1.
About half of the patients were previously treated, and about half were treatment-naïve. At the investigators’ discretion, patients were allowed to receive injections of Lucentis (ranibizumab, Genentech) starting at 2 weeks after ICON-1 injection.
One serious adverse event and one severe adverse event occurred. Ocular adverse events were mild to moderate, Regillo said.
A single dose of ICON-1, alone or followed by ranibizumab injections, showed potential dose-related signals of biologic activity.
Enrollment has begun for the phase 2 EMERGE study, which will evaluate the safety, efficacy and pharmacokinetic profile of repeated ICON-1 injections alone and in combination with ranibizumab compared with ranibizumab alone in eyes with treatment-naïve CNV related to AMD.
The EMERGE study will include 90 patients randomized to three arms: a 300-µg injection of ICON-1 plus sham injection (30 patients), a 300-µg injection of ICON-1 plus 0.5-mg ranibizumab (30 patients) and 0.5-mg ranibizumab plus sham injection (30 patients).
|
 |
Anti-complement C5 monotherapy ineffective in reducing geographic atrophy lesion size
An anti-complement C5 therapy had no different effect on geographic atrophy lesion size than sham therapy, according to a presentation at Angiogenesis, Exudation, and Degeneration 2016
The multicenter phase 2 trial included 150 patients with bilateral age-related macular degeneration and a geographic atrophy (GA) lesion in the study eye between 3 mm2 and 16 mm2 in area at baseline.
Patients were randomized 2:1 to receive intravitreal injections of 5 mg per month of the study drug or monthly sham treatment in the study eye. Primary endpoint was mean change in GA lesion area at month 12 as assessed by fundus autofluorescence. There was no statistically significant treatment difference observed.
Ninety-nine patients were in the LFG316 group; 51 patients were in the sham group. There were no major imbalances between the groups with regard to age, gender, body mass index, or years of AMD.
With regard to safety, there were no serious adverse events or non-ocular adverse events related to the study drug, Zamiri said.
“A couple of patients with intraocular inflammation and a few patients with raised IOP were similar to [the rates] we’ve seen previously with intravitreal injections,” Zamiri said. The rate of endophthalmitis was also similar to that associated with intravitreal injections, Zamiri said. Visual acuity gains were nominal, again with no significant difference between LFG316 and sham, she said.
“LFG316 has an acceptable safety profile ... for further development, but monthly treatment with LFG316 monotherapy was not efficacious, either in GA lesion reduction or in visual acuity [gains],” Zamiri said
|

MEET
THE FELLOWS
In each issue, a Fellow of the Optometric Retinal Society is highlighted. In this issue, Dr. Anna Bedwell, a new ORS fellow, will be highlighted
Dr. Anna Bedwell is a graduate of Indiana University for both her undergraduate and optometry degrees. She completed her residency at the San Francisco VA Hospital in 2011. Since residency, she returned back to Indiana University and joined the faculty as a now Clinical Assistant Professor. Her main teaching assignment is at the school’s satellite clinic in Indianapolis where she works with 4th year optometry interns. She sees everything including ocular disease, primary care and contact lens but has a particular interest in posterior segment disease. She is co-supervisor of the clinic’s primary care residency which is in its first year. Outside of her clinic duties, Dr. Bedwell enjoys giving continuing education lectures and submitting posters. She has been a fellow of the American Academy of Optometry since 2013.
Dr. Bedwell resides in Indianapolis with her husband and infant daughter. When not changing diapers, she would love to be reading, walking with her Labrador retriever or enjoying a glass of wine.
WHY
BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us. |

SPONSOR NEWS


Editor
in Chief
Steven Ferrucci, OD, FAAO
Co-Editor
Mark T. Dunbar, OD, FAAO |
Journal
Reviewers
Tahrim Rahman, OD
Kristie Draskovic, OD
Savannah Brunt, OD
Nicholas Zagorianos, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|