| |
Volume 13, Number 4 |
December
2017 |
|
Inside
This Issue |
|
|
|
| This e-newsletter is provided free to doctors through industry support from |
 |
|
FROM
THE DESK OF THE EDITOR
As the end of 2017 is soon approaching, I can honestly say I am hoping for a much better year in 2018. This year, particularly the last three months, have been rough on myself and family. My father unexpectedly passed away in October at the young age of 61.
Among the many influences my father had on my life, two stick out the most: introducing me to optometry and Indiana University (Go Hoosiers!). I grew up in a small town in southern Illinois where my father was the only optometrist in the whole county. Much of what I learned about patient care was from my dad. He taught me how to treat patients respectfully and most importantly, to listen. He made sure to spend plenty of time with each patient (his staff would often say too much time). Because he practiced in a rural area, he was always looking out for the out-of-pocket costs for his patients. Optometry itself was never his passion. It was taking care of people that mattered to him the most. Watching him practice optometry, I developed an interest in the career and ended up following suit.
After I graduated from optometry school in 2010 at IU, our roles reversed and he began to seek my advice instead. He called on me when the Illinois law added orals and when he was ready to purchase his first OCT. We talked as colleagues though he still gave unsolicited advice. He had one more year left until he would be fully retired and had already started new projects, including starting a new business, a brewery, from the ground up. Even though I did not go into practice with him, he was very proud of my career path.
Dr. William “Kip” Bedwell was an optometrist that very few of you reading this knew. However, the way he practiced optometry is something we should all strive for. Listen to what your patients are saying, relate to them, and understand their point of view: care not just for their eyes, but for them as a people.
Anna Bedwell, OD, FAAO
Editor-in-Chief
 |
PRESIDENT'S MESSAGE
Our annual meeting, ORS Retina Update 2017, was held in conjunction with Review of Optometry December 1st and 2nd in Anaheim, Calif., and was a great success. Over 100 doctors as well as 16 sponsors joined us to make it extra special. Thanks to all the sponsors, without whom the meeting would not be nearly as successful. That includes Alcon, Annidis, Banyan, Centervue, EyePromise (formerly ZeaVision), Heidelberg, Maculogix, MacuHealth, Novartis, Nidex, Optos, Optovue, Regeneron, Spark Therapeutics, Quantel Medical and Zeiss. Also, a special thanks to Rishi Singh, MD, from the Cleveland Clinic, who provided top-notch education, both in a panel with Dr. Mark Dunbar and myself on AMD, as well as the keynote lecture on the surgical management of VMT and macular holes. His contributions were phenomenal, and he was an absolute joy to work with. I also want to thank Jobson and Review of Optometry for helping with the logistics of the meeting, most notably Casey Foster, Mike Hoster, Jonathan Dardine and Amy Hellem. Without them, it would have been a train wreck. I also want to thank Mark Dunbar, who offered his expertise (or what little he has), to the program with several great lectures over the weekend. I also need to thank my only friend Mo Rafieetary for coordinating the education, and coming up with a great lineup of lectures. Lastly, thanks to all the attendees who came out. Without you, this would all be for nothing, as it is truly the mission of the Optometric Retina Society to promote the advancement of vitreoretinal knowledge for clinicians, ophthalmic educators, residents, and students. I truly hope you enjoyed the meeting and would love to see you again when we do this next year in 2018. Stay tuned for details, exact dates, and location!!
I wish each and every one of my fellow ORS fellows as well as readers of this newsletter and their families a healthy, safe, and prosperous holiday season and New Year!! Here’s to a great 2018!!
Sincerely,
Steven Ferrucci, OD, FAAO
ORS President

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.
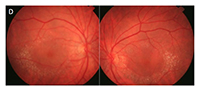
Figure 1.
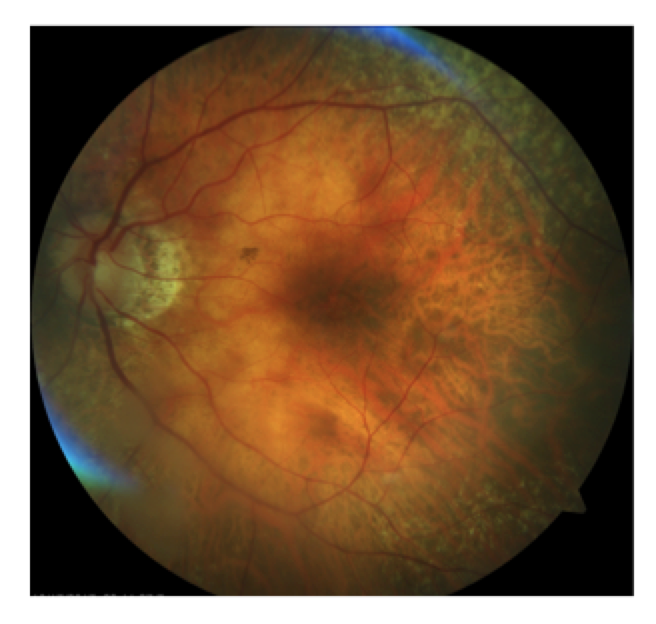
Figure 2.
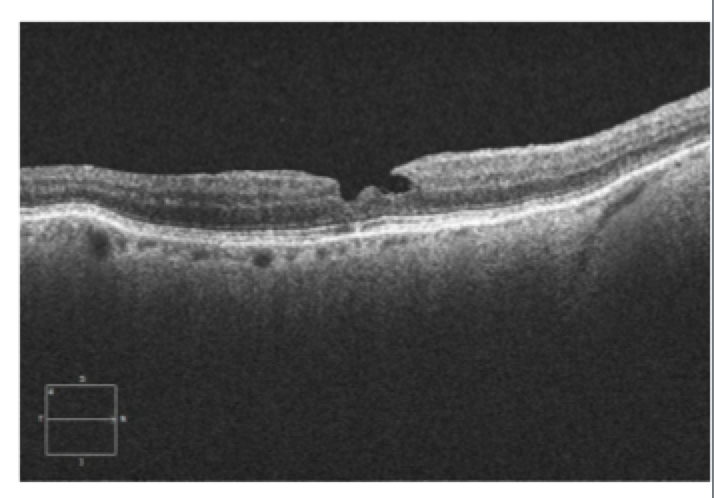
|
Answer appears later in newsletter. 
Image Gallery
Which of the following image pairs represents Canthaxanthin retinopathy?
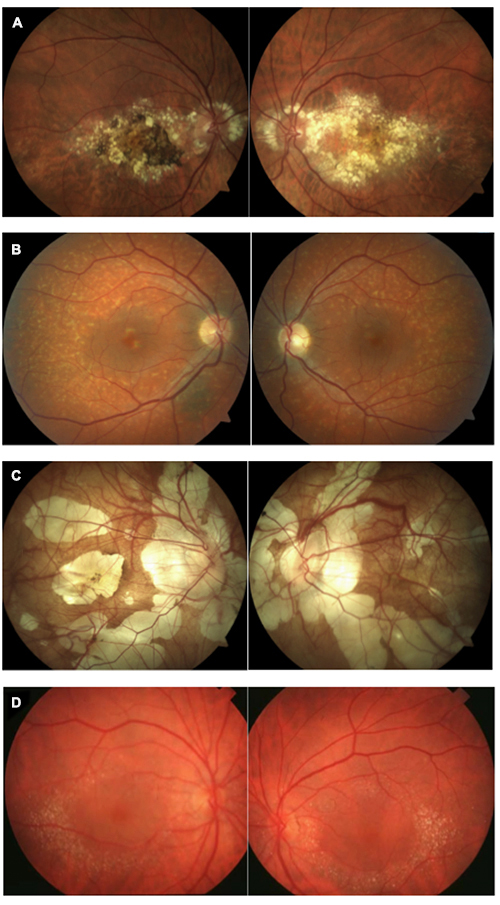
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Choroidal Thinning Associated With Hydroxychloroquine Retinopathy
Hydroxychloroquine (HCQ) is a common drug utilized in the treatment of rheumatologic and dermatologic conditions. Though the drug is regarded as safe, there is a risk of HCQ retinopathy. This study assessed the effect of HCQ on the choroid. This was a retrospective review of 146 patients diagnosed with either systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) and on HCQ. Those with high myopia, recent use of oral steroids or concurrent macular or retinal disease were excluded. Of those, 124 met the criteria for inclusion. All patients had a comprehensive dilated examination including swept-source OCT imaging (all taken in the morning hours in a three hour range). Choroidal and choriocapillaris-equivalent thicknesses were measured at 0.5 mm, 1.5 mm and 3 mm nasal and temporal to the fovea. Patients were evaluated for HCQ retinopathy following the AAO established guidelines. The duration of HCQ use among patients varied from six months to 20 years (mean 8.6 years). Twenty (16.1%) of the patients demonstrated HCQ retinopathy.
There was a significantly thinner choroidal thickness in the eyes with HCQ retinopathy (all p<.05) except in the area 1.5 mm temporal to the fovea. As well, the eyes with HCQ retinopathy had a significantly thinner choriocapillaris-equivalent thickness at all locations. Conversely, the medium-to-large vessel layer thickness was only significantly different at a few locations. There was much more choroidal thinning noted in areas with corresponding outer retinal defect. Eyes with more severe HCQ retinopathy had more choroidal thinning than those with mild retinopathy.
No previous papers have discussed the impact of HCQ on the choroid. This study is limited based on the retrospective nature and small sample size, particularly in the HCQ retinopathy group. Further study of the choroid in HCQ retinopathy may help us to better understand the mechanism of HCQ toxicity and improve clinical evaluation for toxicity.
Ahn SJ, Ryu SJ, Joung JY, et al. Choroidal thinning associated with hydroxychloroquine retinopathy
Am J Ophthalmol 2017;183:56–64.
A Retrospective Study of the Influence of the Vitreomacular Interface on Macular Oedema Secondary to Retinal Vein Occlusion
It has been demonstrated in neovascular AMD that vitreomacular adhesion (VMA) or traction (VMT) can antagonize the effect of anti-VEGF treatment. This study sought to analyze the relationship between anti-VEGF treatment for macular edema secondary to retinal vein occlusion (RVO) and the status of the vitreoretinal interface. This was a retrospective review of patients with treatment-naïve eyes with RVO treated with anti-VEGF injections. All patients presented with a new diagnosis of RVO between January 2011 and June 2014. Patients with prior intravitreal injection treatment, uncontrolled glaucoma, ERM, neovascular AMD, prior vitrectomy or vitreolysis injection were all excluded. Based on OCT, 114 eyes were categorized using the international classification system for VRI disorders. Three groups were made: VMT (group A), no PVD (group B) and clinical diagnosis of PVD without VMA/VMT on OCT (group C). Of the 114 eyes, 52 of those met the criteria (group A=15, group B=24 and group C=13).
Mean central subfield thickness (CST) across all groups was 483.0±179.9 um. At six month follow up, the reduction in CST was −224.13 μm in group A, −160.88 μm in group B and −50.92 μm in group C (p=0.11 between cohorts). Between groups the final CST at six months was comparable: 306.21 μm in group A, 327.39 μm in group B, and 328.25 μm in group C (p=0.80 between cohorts). Additionally, regardless of VRI status, the change in BCVA at month six was statistically comparable.
Previous studies, prior to OCT, suggested that vitreous traction was responsible for the persistent macular edema in RVO. It was theorized that VMT would hinder improvement from anti-VEGF injections. However, in this study, eyes with VMT actually showed better gains in BCVA and a superior decrease in CST. It is unlikely the PVD played a role as the VRI classification did not change for any patient when checked at six months. A larger, prospective is necessary to confirm the findings, as this study was limited by sample size and confounding factors such as lens status.
Singh RP, Habbu KA, Bedi R, et al. A retrospective study of the influence of the vitreomacular interface on macular oedema secondary to retinal vein occlusion. Br J Ophthalmol 2017;101:1340–5.
Comparison of Visual Outcomes in Coats’ Disease: A 20-Year Experience
Coats’ disease is an idiopathic retinal vascular abnormality characterized by retinal telangiectasia and lipid exudation and is traditionally treated with cryotherapy or laser photocoagulation. This retrospective cohort study looked at visual acuity outcomes among Coat’s disease patients who sought treatment at a tertiary-care, university-based practice.
Thirty nine eyes of 39 patients were divided into two categories to compare treatment approaches and outcomes over two distinct 10 year periods: decade one (1995-2005) had 19 eyes and decade two (2006-2015) had 20 eyes. Management methods included observation, cryotherapy, vitreoretinal surgery, intravitreal triamcinolone, or enucleation. Selected eyes after 2008 were given off-label intravitreal bevacizumab (IVB) based on treating physician preference. Only one eye from each patient was included, and the better eye from patients with bilateral disease was excluded.
One trend showed decade one eyes as having more advanced stages of disease than decade two suggesting earlier detection of disease over time. This may be due to improvements in earlier visual screenings in children and better technology in screening for retinal disease. Mean visual acuity worsened from initial to final visit in decade one eyes whereas the mean visual acuity for decade two eyes remained stable. More advanced stages of disease were found in decade one than decade two and may explain the worse visual outcomes for decade one. Decade one had more observation than treatment than decade two. This may indicate a shift away from observation due to increasing evidence showing aggressive treatment of Coats’ achieves better resolution of the disease. There was no difference in tractional retinal detachment and macular scarring development between decades one and two eyes treated with ablation only vs. ablation with IVB, and primary intravitreal surgery with ablation vs. primary intravitreal surgery with ablation and IVB.
This study suggests that disease detection has improved over time, resulting in better visual outcomes with aggressive treatment than earlier decades.
Ong SS, Buckley EG, McCuen BW 2nd, et al. Comparison of visual outcomes in coats’ disease: a 20-year experience. Ophthalmol 2017;124(9):1368-1376.
Associations Between Individual Retinal Layer Thickness and Diabetic Peripheral Neuropathy Using Retinal Layer Segmentation Analysis
Diabetes mellitus (DM) is known to cause retinal and nerve complications. Previous studies have found a correlation between corneal nerves and the ophthalmic division of cranial nerve V with diabetic peripheral neuropathy (DPN), implying that a nerve abnormality in DM may be associated with both central and peripheral nerves. This retrospective, observational, cross-sectional study looked at macular nerve fiber layer thickness in 120 eyes of 120 patients separated into three categories: normal controls, subjects with DM and no DPN, and subjects with DM and DPN.
All subjects had SD-OCT scanning of the macula using Spectralis OCT and perifoveal volumetric retinal scans including 25 single, horizontal axial scans. Newly introduced segmentation technology separated the retina into individual layers and their thicknesses were measured.
There were statistically significant differences in the mean thickness of the retinal nerve fiber layer (RNFL) and photoreceptor layer among the three categories. The average RNFL thickness was less in subjects with DPN than the normal control group and diabetics without DPN. The ratio of mean RNFL thickness to mean total retina layer was less in subjects with DPN. Also, mean thickness of the photoreceptor layer was less in diabetic subjects with and without DPN than the normal control group, which may indicate worse vision in patients with DM than normal subjects. No other retinal layers had a significant difference in thickness between the three groups.
Retinal layer thicknesses were also compared between those with DM and diabetic retinopathy (DR) vs. subjects with DM and no DR. The thickness of RNFL was less in DM patients with DR than those without DR. It was also found that the presence of DPN increases with longer diabetes duration, and a decrease in RNFL and INL thicknesses were found to increase the likelihood of developing DPN. This study provides clinical evidence that degenerative changes in the peripheral nerves of diabetic patients seems to occur along with structural changes in RNFL.
Kim JH, Lee MW, Byeon SH et al. Associations between individual retinal layer thickness and diabetic peripheral neuropathy using retinal layer segmentation analysis. Retina. 2017; Sep 7. [Epub ahead of print].
Efficacy of Intravitreal Aflibercept in Macular Telangiectasia Type 1 is Linked to the Ocular Angiogenic Profile
Idiopathic macular telangiectasia (MacTel) type 1 presents with microvascular changes temporal to the macula. This most often presents unilateral in middle-aged males. Traditionally, anti-VEGF treatment with bevacizumab and ranibizumab has worked inconsistently in reducing the macular edema associated with MacTel type 1. This is a small, retrospective case series evaluating the effect of aflibercept treatment in patients with macular edema caused by MacTel type 1.
Eight subjects had MacTel type 1 and eight were healthy controls. The subjects with MacTel type 1 all had macular edema with a history of laser photocoagulation, and in seven of those, also anti-VEGF therapy (bevacizumab or ranibizumab). The patients with MacTel type 1 received two initial monthly loading doses of intravitreal aflibercept and then were dosed as needed on four-week intervals. Over the 12-month study, the patients received an average of 6.6 ± 1.4 intravitreal aflibercept injections.
Visual and anatomic improvement was found with aflibercept treatment. A reduction in central macular thickness (CMT) was noted in all patients, decreasing from 434 ± 98 μm at baseline to 293 ± 59 μm (p=0.014). The BCVA improved in 7/8 patients. In total, the acuity improved from 79.6 ± 16.3 ETDRS letters to 88.0 ± 11.2. Aqueous humor samples were taken and compared between the eight controls and 6/8 MacTel type 1 patients. There was no difference in VEGF-A levels (x1.3; p=0.95) but considerably higher levels of sFlt-1 (x4.3; p=0.013), P1GF (x2.2; p=0.029), and Tie-2 (x3.7; p=0.019) and VEGF-D (x6.8; p=0.049) in the MacTel group.
In this study of MacTel type 1, patients refractory to previous laser or bevacizumab or ranibizumab responded very favorably to intravitreal aflibercept. Bevacizumab and ranibizumab are known to block only VEGF-A, while aflibercept inhibits both VEGF-A and P1GF. This correlated with higher amounts of P1GF detected in the aqueous of MacTel type 1 patients. Though this was a small cases series, intravitreal aflibercept may be a better treatment option especially in those with poor success from laser or bevacizumab/ranibizumab.
Kowalczuk L, Matet A, Dirani A, et al. Efficacy of intravitreal aflibercept in macular telangiectasia type 1 is linked to the ocular angiogenic profile. Retina. 2017; 37:2226–37.
Gene Therapy in Retinal Disease
As eye care providers, sometimes we must simply look a patient in the eye and tell them that we have no treatment options available that can improve their vision. Certainly, this has often been the case with many inherited retinal diseases. We can maximize the individual’s remaining functional vision through low vision rehabilitation, and we can teach them coping skills to enhance their daily lives. Traditionally, however, we have been unable to offer any effective medical or surgical intervention.
Thankfully, we may be on the precipice of beginning to break through that barrier. Currently, there are over 18 gene therapy trials ongoing for various inherited retinal diseases, as well as one for the treatment of Leber’s hereditary optic neuropathy. Notably, Philadelphia-based Spark therapeutics recently received a unanimous FDA committee panel approval recommendation for its gene therapy Luxturna. Luxturna represents gene therapy in the truest form, as it utilizes a viral vector to introduce a healthy copy of the diseased RPE 65 gene that leads to approximately 8.5% of Leber’s Congenital Amaurosis (LCA) cases. Overall, LCA occurs in approximately 2.5 / 100,000 live births. The RPE 65 gene makes a protein that is a vital component of the visual cycle, and it has also been implicated in certain forms of retinitis pigmentosa (RP), accounting for approximately 2% of all RP patients. There are believed to be up to 2,000 individuals in the United States suffering from inherited retinal disease caused by a defective RPE 65 gene, almost all of whom will eventually be totally blind without intervention.
Like many rod-mediated conditions, initial symptoms include night blindness and peripheral vision loss at an early age, followed by further progressive loss and diminishing central vision. While patients in the Luxturna trials did not regain completely normal vision, they did experience substantial improvement in functional vision, including the ability to mobilitate far more effectively, recognize faces, and see the stars in the sky. In fact, several of the individuals in the clinical trials appeared before the FDA committee to testify regarding the therapy’s tremendous impact on their lives. One of them was recently a contestant on America’s Got Talent. Admittedly, several questions remain. It is not known how permanent the positive effect of the therapy will be, but several of the trial participants have retained its benefits for four or more years to date. Also, while the cost of the therapy is not yet known, it will likely be very expensive, with some analysts predicting that it could be in the $750,000 to $1 million range. Finally, a unanimous committee recommendation of approval does not guarantee final FDA approval of the therapy, but typically, the FDA does follow that advice.
The deadline for a final decision is mid-January of 2018. In the meantime, Spark has partnered with Prevention Genetics to offer a truly tremendous program completely free of charge. Known as “ID your IRD,” the program makes free genetic testing available to certain individuals suffering from inherited retinal diseases. Saliva is collected in the doctor’s office and sent to the lab, with results returning in four to six weeks. The genetic panel can identify certain rod-mediated conditions such as LCA, RP, and choroideremia. By testing patients with suspicious findings, individuals can obtain a very specific diagnosis of their condition. The free program even includes a follow-up session with a genetic counselor. Any eye care practitioner interested in more information regarding obtaining the in-office test kits can call 855-772-7589.
Brad Sutton, OD, FAAO
Immediate Past President, Optometric Retina Society

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
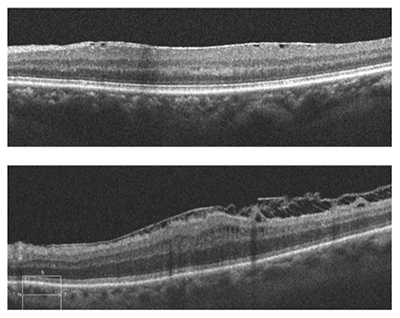
This patient, a 62-year-old female presented with complaints of decreased vision in the left eye for the past three months. Her current best-corrected vision is OD 20/20 and OS 20/60 with no improvement with pinhole. She has a history of cataract surgery, both eyes, two years ago, and her vision at her last exam seven months previous was 20/20 OD and OS. Anterior segment appeared normal and the posterior capsule was clear in both eyes. Her left macula showed evidence of a subtle macular hole (photos above) that was imaged with OCT and proved to be a partial thickness macular hole with associated epiretinal membrane (ERM) (photos below).
ERM is a collagenous fibrocellular proliferation on the inner surface of the retina. ERM can occur idiopathically or be due to many different ocular disease states such as: retinal venous occlusion, retinal laser procedures, retinal detachment, retinal or vitreous inflammation, and posterior vitreous detachment (PVD). The prevalence of idiopathic ERM increases with age and reaches 12% to 20% after age 70. The most common occurrence is following a PVD. As the vitreous detaches from the retina, the light adhesion in the macular area can result in small tears in the inner limiting membrane (ILM). It is theorized that these tears stimulate Müller cells within the retina to transdifferentiate into fibroblasts and myofibroblasts. ERM is a glial fibrillary acidic protein-positive gliotic and fibrotic scar containing various types of collagen and differentiated Müller cells. ERM can have sparse or dense cellular proliferation with surgical removal being more difficult in those cases with more dense cellular proliferation.
The tangential traction of the ERM can distort the normal structure of the retina, giving rise to the common complaint of distorted vision. Increased traction can cause the formation of retinal folds, cysts, and macular holes. Surgical treatment of ERM involves vitrectomy, removal of the membrane and often peeling of the ILM. The inner limiting membrane provides a scaffold for growth of the epiretinal membrane and is much more rigid than the underlying retina. Removal of the ILM provides complete removal of collagen forming cells and allows the more pliable retina to return to its normal architecture.
The postoperative requirement of face-down positioning for macular hole surgery has been a major drawback and limitation for many patients. Macular hole surgery is effectively the same as ERM surgery with the addition of gas tamponade of the foveal area to prevent liquified vitreous from keeping the hole open and thus allowing closure of the hole and a return to more normal retinal architecture. Face-down positioning has been a common practice for macular hole surgery due to the fact that a small gas bubble requires face-down positioning to make certain that the bubble covers the hole and the vitreous does not enter the hole, which would prevent its closure. Newer surgical techniques utilize a much larger gas bubble (95% fill) that do not require face down positioning. Studies have shown little if any difference between large and small bubble procedures, and this has given many patients the option of surgical repair of macular hole that was previously unavailable to them due to their inability to maintain face down positioning for five to 10 days.
Jeffrey Austin, OD, FAAO
ORS Vice President
|

IN THE
NEWS
 |
Phosphorus Adds Ophthalmologic Genetic Tests
Phosphorus is a computational genomics company that offers genetic tests in a broad range of clinical areas and builds software for lab testing. Until now, their focus had been cardiology, oncology and reproductive health. The company announced in September their plan to add two advanced genetic tests to its collection: an ophthalmologic test for inherited vision conditions and a pharmacogenoic test to tailor individualized treatment based on patient’s genetic profile. The new test will be able to detect 146 genes associated with retinal disorders such as achromatopsia, Bardet-Biedl syndrome, cone-rod dystrophy, congenital stationary night blindness, Leber’s congenital amaurosis, various macular dystrophies and RP. Additionally, it will test for 38 genes associated with cataracts, 18 genes associated with glaucoma and eight genes related to corneal dystrophy.
|
 |
First Orion Human Clinical Study Set to Start After Obtaining Full FDA Approval
Second Sight Medical Products, a developer and manufacturer of visual prosthetics, announced in November that the FDA has given full approval to start the Orion™ Cortical Visual Prosthesis System feasibility clinical study. This permits two US sites, UCLA and Baylor College of Medicine, to enroll up to five total patients. The Orion system converts images from a spec-mounted video camera into a sequence of electrical pulses. The pulses are wirelessly transmitted to electrodes inserted on the visual cortex surface. This system allows the retina and optic nerve to be completely circumvented. Thus, it has the potential to restore vision to patients with almost any form of complete blindness.
|
 |
Lin BioScience Obtains FDA Orphan Drug Status in the Treatment of Stargardt’s Disease
Lin BioScience announced in October that LBS-008 received Orphan Drug Status from the FDA to treat Stargardt’s disease. The disease is caused by a mutation in the ABCA4 gene, which leads to excess formation and accumulation of vitamin A dimers in the retina. Toxic vitamin A buildup ultimately progresses to retinal cell death and loss of vision. LBS-008 is an oral treatment that aims to reduce the excessive toxins. It specifically targets retinol binding protein (RBP4) and retinol within the circulation, thus preventing excessive uptake into the eye. Phase I clinical trials for LBS-008 are anticipated to start in 2017 for the treatment of both Stargardt’s disease and dry AMD.
|
 |
Aerie Pharmaceuticals Acquires Rights to PRINT® technology
Aerie Pharmaceuticals, known for its development of glaucoma therapies, announced in October that the company acquired from Envisia Therapeutics the rights to PRINT® technology. PRINT® technology is a system able to manufacture sustained release products. Aerie plans to use this to produce implants containing their product, AR-13154, to treat wet AMD. AR-13154 blocks Rho kinase and protein kinase C. Aerie also acquired Envisia’s intellectual property rights to their pre-clinical dexamethasone steroid product candidate, ENV1105, that likewise uses PRINT® technology. Aerie will compensate Envisia with $25 million in cash and Aerie stock and the prospective to earn more if products reach goals.
|
 |
Pixium Vision to Start Clinical Trial of PRIMA Miniaturized Retinal Implant
Pixium Vision, a developer of bionic vision systems, received authorization from the French regulatory agency to start a clinical trial of five patients with advanced dry AMD using their subretinal implant, PRIMA. PRIMA utilizes 378 electrodes in a micro photovoltaic implant of just 2x2 millimeters in size and only 30 microns thick. The PRIMA implant is surgically placed in a subretinal position. Patients wear spectacles furnished with a camera. The camera converts images to near-infrared light and wirelessly transmits to the implant. The implant converts this into electrical current to stimulate bipolar cells so that communication can be made to the optic nerve and brain. This study is expected to start in 2017 and end in 2022.
|
 |
Graybug Vision Initiates Phase I/II Trial of GB-102 for AMD
Graybug Vision, based in Redwood City, CA, is a pharmaceutical company committed to therapy for vision threatening eye disease. They have started the first clinical trial, ADAGIO, for GB-102, an intravitreal injectable drug to treat neovascular AMD. GB-102 is formulated with a tyrosine kinase inhibitor (sunitinib malate) that can block VEGFR-1,2,3 in addition to other growth factors associated with choroidal neovascularization. It has the potential for twice a year dosing for neovascular AMD. ADAGIO is a two-part study. Part one will study patients switched from IV anti-VEGF therapy to GB-102 alone. Part two will compare GB-102 head to head with standard regiment treatment with aflibercept (Eylea®).
|

|
IMAGE QUIZ ANSWER
Canthaxanthin is a naturally occurring chemical used as an oral bronzing/tanning agent (not FDA-approved in the US) and as food coloring. It is a carotenoid found in crustaceans, pink fish, some mushrooms and some birds. Canthaxanthin deposits in the dermis and has been used occasionally to treat Vitiligo. In high oral doses, canthaxanthin can cause retinopathy characterized by a ring of yellow crystals around the macula as seen in these photos. Retinopathy is dose-dependent, presenting in half of patients taking a daily dose of 37 g. This rises to 100% with ingestion greater than 60 g daily. Luckily, the prognosis for these patients is good as the retinopathy is reversible by discontinuing canthaxanthin. The crystals reabsorb slowly over many years.
A: Doyne’s honeycomb dystrophy B: fundus flavimaculatus C: pathological myopia
|
LARRY ALEXANDER CASE REPORT CONTEST
The ORS is pleased to announce that the Larry Alexander Case Report contest will be available for its second year. The contest is open to optometry residents. Case reports, relating to vitreoretinal disease, will be due in spring 2018. The award will be $1,500, sponsored by Optovue. The winning case report will be published in Review of Optometry. Stay tuned for more information.
|
|
MEET THE FELLOWS Dr. Jim Williamson serves as a staff optometrist and supervisor of six optometric residents at the Veterans Affairs (VA) Medical Center in Memphis. Before graduating from Southern College of Optometry (SCO) in 1997, he received a degree in public relations/journalism, and worked as a caddy for the Australian golf tour.
After completing a hospital-based VA residency in 1998, Dr. Williamson joined the faculty at SCO. A year later, he accepted a position at a referral center. He missed teaching, however, and re-entered the VA in 2002.
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
SPONSOR NEWS


Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Katelyn Lucas, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|