| |
Volume 13, Number 3 |
September
2017 |
|
|
Inside
This Issue |
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
It is extremely rare to see an optometrist-led study get published in an ophthalmology journal, particularly in a retinal journal. So in this issue of our quarterly newsletter I would like to draw your attention to the VAST Study Group, led by Dr. Julie Rodman, whose paper on the prevalence of vitreomacular adhesion will be published in Retina later this year. You can read a summary of the recently-released epub in the abstract section below (though I highly suggest reading the paper in full). Not only is this group led by an OD, but all of the authors are ODs. Additionally, all the authors are fellows of the ORS (just another great reason to consider applying for ORS fellowship). Congratulations VAST group!
Anna Bedwell, OD, FAAO
Editor-in-Chief
PRESIDENT'S MESSAGE
I like technology. I like my iPhone. I like my iWatch. I like my smart TV. Optometry-wise, I like my Optovue Avanti OCT with Angio Vue, as it allows me a way to evaluate the underlying vascular status of an eye without having to inject a dye. It’s faster, less expensive and certainly easier for the patients. I have found it useful with a myriad of conditions, most notably to evaluate CNVMs and to look for macular ischemia in my patients with diabetes. I can see a day when this technology could make traditional dye-based FAs a thing of the past. Likewise, I love my other newest piece of technology, my Eidon True Color Confocal Scanner by Centervue. This is an imaging system that allows fantastic image quality, even through small pupils. I used to say that fundus photos were merely a way to document my findings, but due to the great resolution and wide-field capabilities, I sometimes find myself noticing subtle changes, such as IRMAs, on the images that I did not see in clinic.
However, I do get mildly concerned, as we rely more and more on technology, that it may supplant our desire to talk to patients, to truly understand their issues and diminish our expertise in doing a face-to-face exam. Obviously imaging, whether photos or OCTs, is great, especially for things such as mild edema or subtle retinal changes, but we should not use it to replace our ability to do a careful exam, to evaluate the macula for any signs of edema, to do a good peripheral retinal exam with an indirect ophthalmoscope, or to evaluate the optic nerve head for thinning or any structural changes associated with glaucoma.
So while I love technology, it cannot and should not replace our common sense and interaction with patients face-to-face. Otherwise, we might as well delegate everything to robots and on-line refractions, making us obsolete. Our knowledge, empathy and decision making will remain superior to any technology available.
Lastly, don’t forget we will be hosting our annual ORS Meeting, RETINA UPDATE 2017, this December 1-2, at the Sheraton Park Hotel in Anaheim, Calif. We will be offering a minimum of 14 hours of exciting and innovative education by ORS members, as well as a world-renowned retinal specialist. Last year’s meeting in Scottsdale, Ariz., was, in my opinion, one of the best meetings I have attended, and I expect the same this year. I will be there, and hope you are too!
Sincerely,
Steven Ferrucci, OD, FAAO
ORS President

YOU
MAKE THE DIAGNOSIS
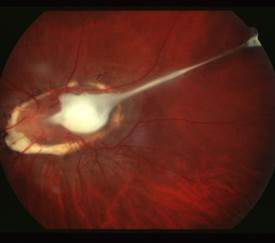
Can you diagnose this condition based on these images?
Figure 1.
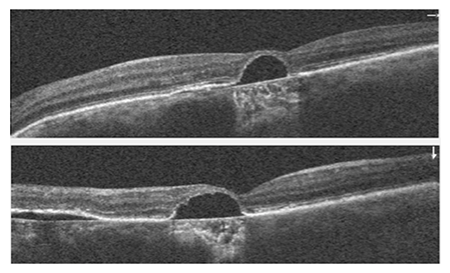
Figure 2.

Figure 3.
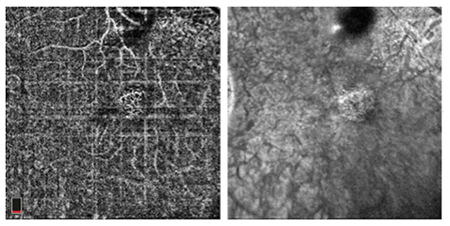
|
Answer appears later in newsletter.

Image Gallery
Which of the following images represents ocular toxocariasis?
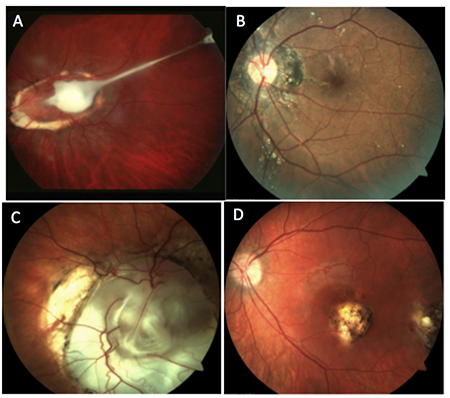
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Prevalence of Vitreomacular Adhesion in Patients without Maculopathy Older than 40 Years
Vitreomacular adhesion (VMA), a partial detachment of the vitreous in the perifoveal macula without corresponding retinal abnormality, can be an incidental finding noted often on OCT. Vitreomacular traction (VMT) can develop from VMA as the vitreous undergoes liquefaction changes. These disorders can be difficult to detect on clinical exam alone. Fortunately, SD-OCT allows a better visualization of the vitreomacular interface and thus a better understanding of these disorders. In this paper, the VAST Study Group sought to find the prevalence of VMA and VMT in patients older than age 40. This was a prospective, cross-sectional study utilizing 14 different centers in the United States that included three academic institutions, five primary eye care clinics and six tertiary private practices. There were 1,950 eyes of 1,090 patients included in this study, all of which had presented for routine eye care. Data obtained included BCVA, Amsler grid testing, fundus biomicroscopy with peripheral retinal exam and SD-OCT. All patients were phakic and did not have pre-existing maculopathy. Of those subjects, the ages ranged from 40 to 89 (mean of 56.2), and about half were women (54.4%). Demographically, 54.4% of participants were Caucasian, 31.1% African American, 9.9% Hispanic and 3.5% Asian or Indian. About half (51.1%) of the studied eyes were hyperopic, 40.1% myopic and 8.8% emmetropic.
Of the 1,950 eyes, the authors found 38.77% had VMA and 1.07% had VMT. Of those with VMA, most (72%) had broad VMA (>1500μm). The odds of having VMA or VMT decreased by 7% for each year of age (p<0.001). Also, they found a 55% reduced chance of VMA or VMT in African Americans as compared with Caucasians (p = 0.025). They did not find an association between VMA/VMT in relation to sex, other races, refractive error, practice site or systemic disease. In subgroup analysis of the patients 63 years and older, VMA was present in 25% and VMT in 2.0%. This agreed fairly well with the Beaver Dam Eye Study, which found a 26% prevalence of VMA in adults ages 63 to 102. A separate subgroup analysis, limited to patients over age 55, was performed to investigate the possible effects of menopause. This analysis found no significant difference in men vs. women having VMA or VMT.
This study provides for clinicians helpful insight into the prevalence of VMA/VMT in the general population. It also raises a new question as to why African Americans would have VMA/VMT less often than Caucasians. As VMA is highly prevalent in middle-aged adults, a better understanding of the vitreomacular interface and the relationship of progression from VMA to VMT is absolutely necessary.
Rodman JA, Shechtman D, Sutton BM, et al. Prevalence of vitreomacular adhesion in patients without maculopathy older than 40 years. Retina. 2017; Aug 10 [Epub ahead of print].
Risk Factors for Recurrences of Central Serous Chorioretinopathy
Central serous chorioretinopathy (CSCR) presents with neurosensory detachments from the retinal pigment epithelium with or without pigment epithelial detachment. It presents most often in middle-aged males. Known risk factors include corticosteroid use, stress and type A personality. This study analyzed recurrence patterns and risk factors for recurrent CSCR. This was a small, single-site, retrospective analysis. The study included patients presenting with active CSCR that fully resolved over follow-up, either spontaneously or with treatment. The patients had to have followed up longer than 12 months after resolution of the first episode. Those that had signs of previous episodes on fundus autofluorescence (FAF) were excluded. Patients with a first episode lasting more than six months or recurrence lasting more than two months were offered treatment with laser photocoagulation or oral mineralocorticoid-receptor antagonist. Patient demographics were recorded in addition to systemic risk factors, including: any type of corticosteroid use, psychological stress, shift work, sleep disorder, depression, allergic disease and cardiovascular risk. Of the 62 patients presenting with CSCR, 46 met the criteria for inclusion. At baseline, patients were imaged with the Spectralis SD-OCT, FAF, fluorescein angiography (FA) and indocyanine green angiography (ICGA). For each patient the degree of leakage on FA was graded by comparing early to midphase. Using a MATLAB algorithm, a measure of leakage intensity between early and midphase was calculated. Three patients were unable to have FA or ICGA due to allergy. Subfoveal choroidal thickness (SFCT) was measured using enhanced depth imaging on SD-OCT. The follow-up on two patients was censored due to receiving oral mineralocorticoid-receptor antagonist for recurrence.
The authors found of the 46 patients, 20 (43%) presented with CSCR recurrence(s) over an average 29.9 ± 9.5 months of follow-up. On average, time from resolution of the initial episode to the onset of recurrence was 7.3 ± 5.5 months. BCVA did not differ between recurrent and non-recurrent cases at initial presentation. However, final BCVA showed a significantly worse acuity in the recurrent group (LogMAR 0.12 vs. 0.03; p=0.07). Risk factors for recurrence in univariate analysis included: SFCT, number of subretinal hyperreflective foci at the leakage site, non-intense fluorescein leakage (moderate, subtle or absent), shift work and irregular PED (near significant, p = 0.093). The authors found in multivariate analysis that recurrences of CSCR occurred more often in patients presenting with thicker SFCT (p = 0.007), non-intense fluorescein leakage (p=0.003), or with a history of shift work (p < 0.0001).
Risk factors for acute CSCR have been well-established. However, those at the highest risk for recurrence has not been widely addressed. Recurrence rates of CSCR vary widely in the literature (20% to 50%), as often patients may not be symptomatic, particularly with non-macular episodes. This study provides insights as to risk factors for recurrence, which include thicker SFCT, non-intense fluorescein leakage, or a history of shift work.
Matet A, Daruich A, Zola M, et al. Risk factors for recurrences of central serous chorioretinopathy. Retina. 2017; May 29. [Epub ahead of print].
Vitreomacular Adhesion or Vitreomacular Traction may affect Antivascular Endothelium Growth Factor Treatment for Neovascular Age-Related Macular Degeneration
Anti-VEGF treatment has been a long-established standard of care treatment for neovascular AMD (nAMD). However, some patients show a poor response to treatment. This paper addresses the impact of the vitreomacular interface in patients’ response to anti-VEGF in nAMD. This is a literature review including nine studies and a total of 2,212 patients looking at the impact of vitreomacular adhesion (VMA) and vitreomacular traction (VMT) on treatment response in nAMD. Studies within this meta-analysis had to meet certain criteria, including: a prospective cohort or retrospective case-control design, receiving anti-VEGF treatment for FA/OCT-confirmed nAMD in the study eye, concurrent VMA or VMT (no VMA/VMT in controls), and a minimum follow up time of six months. All studies had record of at least one of three outcomes: BCVA change from baseline, mean central retinal thickness (CRT) change from baseline or average number of injections over 12 months.
Results found that concurrent VMA or VMT decreased the effectiveness of anti-VEGF treatment in nAMD patients. The average BCVA improvement in the VMA/VMT group was less than in the control group (nAMD without VMA/VMT), particularly at six months (p=0.0002) into treatment and then less so at 12 months (p=0.05). At six months, the mean CRT decrease in the VMA/VMT group was 15.53μm vs. the controls at 32.98μm (p<0.00001). On average, the VMA/VMT group needed more injections than the controls.
Based on the analysis, VMA or VMT adversely affected the efficacy of anti-VEGF injections for nAMD, particularly during the first six months of treatment. The results suggest that the tractional force of VMA/VMT may produce local inflammation, decrease oxygen diffusion, cause local ischemia and disrupt choroidal supply to the macula—all of which could negate the effect of anti-VEGF or promote nAMD. Fortunately, this analysis showed that the unfavorable effect decreased over the treatment period, likely as VMA/VMT naturally released. These results could be applied clinically to understand that nAMD patients with VMA/VMT may initially show minimal BCVA or CRT improvement with anti-VEGF and need additional injections.
Xie P, Zheng X, Yu Y, et al. Vitreomacular adhesion or vitreomacular traction may affect antivascular endothelium growth factor treatment for neovascular age-related macular degeneration. Br J Ophthalmol. 2017; Aug 1. [Epub ahead of print].
Baseline Factors Associated with Visual Acuity and SD-OCT Outcomes in Patients Treated with Intravitreal Anti-VEGF Therapy for Macular Edema due to CRVO or HRVO in SCORE 2
The Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) determined that bevacizumab is noninferior to aflibercept in treating macular edema secondary to central retinal vein occlusion (CRVO) or hemiretinal vein occlusion (HRVO). The intention of this article was to determine what baseline factors are associated with six-month visual acuity and central subfield thickness (CST) outcomes in eyes with macular edema due to CRVO or HRVO treated with bevacizumab or aflibercept.
In this multivariate analysis of the SCORE2 randomized clinical trial, younger age and lower baseline VA letter score (VALS) were associated with better VA outcomes at month six. It is hypothesized that younger patients’ photoreceptors have an enhanced resilience and may permit the retina to recover after a retinal vein occlusion. A lower baseline VALS associated with a greater improvement from baseline is likely due to a ceiling effect; starting from a higher baseline VALS leaves less room for VA improvement with anti-VEGF therapy than starting from a lower baseline VALS. SCORE2 also found that aflibercept was associated with higher odds of macular edema resolution and a CST less than 300μm compared with bevacizumab, but not better VA outcomes at the six-month mark. This may be due to aflibercept’s broader mechanism of action, tighter binding affinity or both. Both aflibercept and bevacizumab inhibit all isoforms of VEGF-A, but aflibercept also inhibits VEGF-B and placental growth factor.
Baseline factors associated with better six-month VA outcomes include younger age and worse baseline VA. Compared with bevacizumab, aflibercept treatment was associated with a CST less than 300μm and resolution of macular edema, but not better VA outcomes. These findings may aid in evaluating monthly anti-VEGF injection responses over a six-month period for patients with macular edema due to CRVO or HRVO.
Scott IU, VanVeldhuisen PC, Ip MS, et al. Baseline factors associated with visual acuity and SD-OCT outcomes in patients treated with intravitreal anti-VEGF therapy for macular edema due to CRVO or HRVO in SCORE 2. JAMA Ophthalmol. 2017;135(6):639-49.
Intravitreal Ranibizumab Injection for Retinopathy of Prematurity
Retinopathy of prematurity (ROP) is a proliferative retinopathy affecting premature infants of low birth weight. The fundamental process underlying the development of ROP is incomplete retinal vascularization. Historically, treatment is done with laser photocoagulation or cryotherapy; however, many studies are emerging using bevacizumab or ranibizumab injection as the primary treatment.
This retrospective study assessed the use of an intravitreal ranibizumab (IVR) injection on infants with ROP. A total of 283 eyes from 145 patients with type 1 ROP or aggressive posterior retinopathy of prematurity (APROP) were injected using 0.25mg/0.025mL IVR. Type 1 ROP is defined as zone I at any stage with plus disease, zone I at stage 3 without plus disease, and zone II at stage 2-3 with plus disease. APROP is an uncommon, severe form of ROP characterized by its posterior location, neovascularization and the presence of plus disease. Out of the 283 eyes, 266 eyes (94.0%) showed a positive response to IVR, and 17 eyes (6%) showed a negative or no response. The negative/no response group progressed further and required a vitrectomy and/or laser to reattach the retina. Patients with a negative/no response had a higher postmenstrual age and postnatal age at the time of injection than the positive response group, indicating that delayed IVR treatment may lead to a negative/no response. The 266 eyes showing a positive response had 139 eyes (48.6%) regress without reactivation and 127 eyes (44.9%) regress with reactivation. The patients who regressed with reactivation post-IVR were retreated using laser and achieved a flat retina. It was found that the likelihood of reactivation after IVR was higher in patients with gestational age ≤29.5 weeks, except for those with zone II stage 2.
IVR appears to be effective in treating ROP; however, the outcome is dependent on gestational age and ROP type. Ranibizumab had a higher reactivation rate compared with bevacizumab studies, but ranibizumab may be safer for premature infants due to its shorter half-life and thus shorter systemic VEGF suppression. Ranibizumab is less time-consuming than laser, as well as less risky since it allows for further retinal vascularization. Although this study had the largest number of patients compared with other ranibizumab studies, there are still questions about appropriate IVR dosing, timing of injection and long-term outcome; thus, further studies are needed.
Huang Q, Zhang Q, Fei P, et al. Ranibizumab injection as primary treatment in patients with retinopathy of prematurity: Anatomic outcomes and influencing factors. Ophthalmology. 2017 Aug;124(8):1156-64.
Trends in Fluoroquinolone Non-susceptibility among Coagulase-Negative Staphylococcus Isolates Causing Endophthalmitis
Endophthalmitis is the most feared potential complication of cataract surgery, with the most common causative agent being coagulase-negative Staphylococcus. Though very rare with modern surgical techniques, the result of postoperative intraocular infection can be devastating. Antibiotic prophylaxis is most commonly carried out with fluoroquinolone antibiotics, most often topically before and after surgery, but sometimes via intracameral injection during the procedure. This study examined non-susceptibility rates of coagulase-negative Staphylococcus causing endophthalmitis over a 22-year period at the Bascom Palmer Eye Institute in Miami from January 1, 1995 to December 31, 2016. Susceptibility rates to ciprofloxacin (second generation), levofloxacin (third generation) and moxifloxacin (fourth generation) were studied. The results were somewhat sobering. Over the time period examined, the non-susceptibility rate for ciprofloxacin increased from 28% to 56%. Levofloxacin exhibited an increase from 17% to 56%, while the rate for moxifloxacin went from 22% to 57%. By the end of 2016, over half of the coagulase-negative Staphylococcus strains tested were not susceptible to these three fluoroquinolones.
There was one small piece of encouragement, however. For all three drugs, the non-susceptibility rates actually peaked from 2005 to 2009, then decreased slightly from 2010 to 2016. Nonetheless, antibacterial therapy continues to present significant challenges, as bacteria continually adapt and mutate to confer resistance to the agents that we design to eradicate them. This study reinforces the pressing need to continue to develop novel agents in our fight against these ancient organisms.
Stringham JD, Relhan N, Miller D, Flynn HW Jr. Trends in fluoroquinolone nonsusceptibility among coagulase-negative Staphylococcus isolates causing endophthalmitis. JAMA Ophthalmol. 2017 Jul 1;135(7):814-15.
Cataracts Induced by Neodymium-Yttrium-Aluminum-Garnet Laser Lysis of Vitreous Floaters
Few entities that are encountered in eye care are as chronically annoying to patients as vitreal floaters. Because no universally-accepted method exists for treating floaters where the benefit is consistently viewed as outweighing the risk, patients are often motivated to pursue somewhat unproven techniques in an attempt to rid themselves of nearly constant aggravation. One of these techniques is YAG laser vitreolysis of symptomatic floaters. A YAG laser is used to lyse the floaters at a power ranging from 2.5mJ to 4.5mJ with a typical upper limit of 500 shots. No large, case-controlled studies exist in the literature regarding the use if this technique, but success is relatively modest, with one recent study reporting that 38% of patients experienced a modest improvement in symptoms. In addition to the debatable success rate, several complications have been reported, including refractory glaucoma. This case report is the first to detail another potential complication: posterior capsular openings and PSC cataracts. Two patients reported after having had YAG laser vitreolysis of floaters performed elsewhere. Both exhibited an opening in their posterior capsule that was also associated with a PSC cataract. The first patient, a 63-year-old white female, presented one week after the procedure with 20/50 BCVA. The second, a 65-year-old white male presented approximately two years after the procedure with 20/70 BCVA. While he waited two years to be seen, he did report that his vision in the eye in question started to decline shortly after the laser surgery was performed. Thankfully, with a combination vitrectomy and cataract surgery with IOL placed in the sulcus, both patients regained 20/15 and 20/20 vision, respectively. While it is clear that persistent floaters can be highly bothersome and even debilitating, we currently lack a treatment method that combines efficacy with a tolerable risk profile, with the possible exception of emerging small port pars plana vitrectomy techniques. YAG laser vitreolysis remains quite risky and, based upon this report, should likely be avoided in phakic patients.
Koo EH, Haddock LJ, Bhardwaj N, et al. Cataracts induced by neodymium-yttrium-aluminum-garnet laser lysis of vitreous floaters. Br J Ophthalmol. 2017;101:709–11.
Correlation Between Short-and Long Term Effects of Intravitreal Ranibizumab Therapy on Macular Edema After Branch Retinal Vein Occlusion: A Prospective Observational Study
The use of intravitreal anti-VEGF agents in the treatment of branch retinal vein occlusion (BRVO)-induced macular edema has become commonplace. When utilizing this management technique, how soon after the initial injection can a judgement be made on the potential long term success of therapy? According to one new study, the answer to that question is very soon. This study enrolled 21 eyes of Japanese patients with macular edema induced by BRVO. For purposes of the study, a hemi-central retinal vein occlusion was considered to be under the general BRVO category. None of the 21 patients had undergone any previous anti-VEGF injections, and none had treatment of any kind for the macular edema during the three months prior to enrolling in the study. All eyes received an intravitreal injection of .5mg of ranibizumab. BCVA and foveal thickness (FT) were measured one day, one month, end then at least every two months until six months after the initial injection. If macular edema recurred during the study period, a repeat injection was administered. The mean number of injections per study eye was two. In addition, two eyes received laser photocoagulation therapy outside of the vessel arcades during the study.
Remarkably, both BCVA and FT thickness improved substantially one day after the initial injection. The LOGMAR BCVA improved from .65 to .51 at day one, with further improvement to .29 at six months. The FT decreased from 482μm to 349μm at one day and 305μm at six months. Significant improvement in BCVA at day one was positively correlated with the BCVA at six months, while a decrease in FT at one day was not. So what does this mean? Essentially, at least in this small number of Japanese individuals, patients who show a significant improvement in their BCVA one day after an initial injection will likely maintain visual improvement at least through six months.
Minami Y, Nagaoka T, Ishibazawa A, et al. Correlation between short- and long-term effects of intravitreal ranibizumab therapy on macular edema after branch retinal vein occlusion: a prospective observational study. BMC Ophthalmol. 2017 Jun 13;17(1):90.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
A 62 year-old Caucasian male presented to the clinic reporting the recent onset of a “fluctuating blind spot” in his left eye. His past ocular history was significant for retinal cysts in both eyes which were never treated, but were diagnosed by a local retinal specialist. His best-corrected visual acuity was 20/25 OD and 20/50 OS. A paracentral scotoma was found OS using Amsler Grid. Anterior segment biomicrosopy revealed age appropriate lenticular changes. The posterior segment examination revealed multifocal central serous chorioretinopathy, significant non-central geographic atrophy of the retinal pigment epithelium and atrophic AMD bilaterally. SD-OCT studies demonstrated a neursensory detachment at the macula with an adjacent flat neurosensory detachment OS. Directly neighboring the central serous detachment was an area of thickening and disruption of the RPE-choriocapillaris complex with adjacent subretinal fluid consistent with choroidal neovascularization. (Figure 1) SD-OCT provided outstanding structural resolution of the lesion, however this technology was not able to delineate subclinical microvascular activity. OCT angiography enabled visualization of the choroidal vasculature and allowed for an in-depth analysis of the CSR and surrounding tissue. Evident on the angiogram was a lacy, glomerular network consisting of tangled newly formed vessels originating at the choriocapillaris consistent with an occult CNVM. (Figure 2,3) Choroidal neovascularization can complicate chronic central serous chorioretinopathy and may be difficult to diagnose because CSC itself can be associated with subretinal fluid and ill-defined patterns of hyperfluorescence on fluorescein angiography. En-face SD-OCT has been used to identify CNV but often the vascular contrast is low, limiting image detail. OCTA provides a novel way to potentially detect CNV. In this case, OCTA identified an occult, Type 1 CNV associated with CSR.
Julie Rodman, OD, FAAO
ORS Fellow
|

IN THE
NEWS
 |
Samsung Bioepis Developing Lower-cost Biosimilar to Lucentis
South Korea’s Samsung Bioepis is projecting to start phase III clinical trials in September for SB11, a biosimilar to Lucentis (Genentech). Bioepis currently produces biosimilars for many autoimmune and oncology medications. Lucentis will lose its patent protection in 2020 in Europe and 2022 in the United States. Also listed in the Bioepis pipeline is a biosimilar to Avastin (Genentech), called SB8, which is currently in phase III clinical trial for treatment of lung cancer.
|
 |
Genentech’s Actemra (Tocilizumab) Approved by FDA for Giant Cell Arteritis
Genentech announced that Actemra, a humanized interleukin-6 (IL-6) receptor antagonist, has received its sixth FDA approval since its introduction in the United States in 2010. This makes Actemra the first therapy approved by the FDA for the treatment of giant cell arteritis. The approval was granted based on results from the GiACTA study. This placebo-controlled, multicenter trial found that subcutaneous injection of Actemra, initially combined with a six-month steroid treatment, more effectively continued remission compared with placebo with a 26-week or 52-week steroid taper.
|
 |
Neurotech Shows Positive Phase II Results in treatment of Macular Telangiectasia
Neurotech Pharmaceuticals released 24-month results of its NT-501 delivering ciliary neurotrophic factor (CNTF) in the treatment of patients with macular telangiectasia (MacTel) type 2. This multicenter, randomized clinical trial showed a decrease in photoreceptor loss in treated eyes compared with sham. This difference in the progression of the MacTel lesion was statistically significant (p=0.030), noting that the ellipsoid zone break enlarged by 0.213mm2 in the sham group vs. 0.148mm2 in the NT-501 group. NT-501 employs Neurotech’s proprietary encapsulated cell therapy (ECT), a drug delivery system that allows for continuous production of therapeutic proteins. A single surgical procedure with a small scleral incision is needed for insertion of the ECT, which can then deliver the medication for a minimum of two years.
|
 |
Allegro Ophthalmics Receives $10.7 Million in Private Financing
Allegro Ophthalmics, a biotechnology company focused on vitreoretinal disease therapy, reported securing $10.7 million in private equity financing. The company’s lead investigational drug, Luminate, has successfully met the endpoints in two phase II monotherapy studies in the treatment of diabetic macular edema and vitreomacular traction. Luminate targets integrin receptors to stop and reduce new blood vessel formation. This additional capital could fund further phase II trials to prepare for a Phase III trial.
|
 |
B&L Receives FDA 510(k) Clearance for Hypersonic Vitrector
Valeant’s subsidiary, Bausch + Lomb, has announced 510(k) clearance from the FDA for Vitesse, a hypersonic vitrector. Traditionally, vitrectors operate like a guillotine. Vitesse, however, is bladeless and uses a single-needle design and continuously open port. The new technology will only be available on the company’s new Stellaris Elite Vision Enhancement System. Thus far, Vitresse has completed two phase I studies, one on cadaveric porcine eyes and the other on a cadaveric human eye. Additionally, it has completed two phase II studies with live porcine eyes. The next step will be a human eye study starting summer 2017.
|

|
IMAGE QUIZ ANSWER
A. This photo shows an optic disc granuloma with a vitreoretinal tractional band into the posterior pole, consistent with ocular toxocariasis. Ocular toxocariasis in humans is caused by infection from Toxocara canis or Toxocara cati. These are roundworms found in either dogs or cats, respectively. Contact with infected puppies/kittens or accidental ingestion of embryonated eggs from contaminated soil or food sources causes the infection in humans, most often young children.
Ocular toxocariasis typically presents unilaterally. In the acute phase, the retinochoroiditis reveals vitritis overlying a hazy, white granulomatous lesion. Once the inflammation clears, the granuloma, located either within the posterior pole or in the peripheral retina, can be better appreciated. Even with treatment, visual prognosis is generally poor.
B: angiod streaks C: coloboma D: toxoplasmosis
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
SPONSOR NEWS


Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Katelyn Lucas, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|