DEWS II: Redefining Dry EyeFollow the links below to read the other articles from our coverage of TFOS DEWS II: A Definitive Decade for Dry Eye New Insights on How Dry Eye Happens |
This article covers the following TFOS DEWS II reports:
I. Definition and Classification
III. Epidemiology
VII. Iatrogenic.
In light of three decades of increasing dry eye disease (DED) awareness, the TFOS DEWS II Definition and Classification subcommittee was tasked with constructing an updated, evidence-based definition and new classification system for DED. [I, abstract, p. 276] During a special session at this year’s Association for Research in Vision and Ophthalmology (ARVO) meeting in Baltimore, Md., subcommittee co-chair Jennifer Craig, PhD, said the main goals of the definition update were “resolving the confusion between the diagnostic vs. pathophysiological features, acknowledging the multitude of etiological triggers that could lead to the perpetuated, vicious cycle we see of events in DED and recognizing the role of neurosensory abnormalities in DED.”
Following a thorough review of today’s collective understanding of DED, the subcommittee arrived at the following revised definition: [I, 4.3, p. 278]
Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.
GUIDE TO NOTATIONThis supplement summarizes the 10 subcommittee reports that comprise TFOS DEWS II, published in the July 2017 Ocular Surface. The top of each article lists the reports discussed therein. Readers interested in more detail are encouraged to seek out the full text. For easier exploring, key points cite the relevant source by report, paragraph and page number. As you read the ensuing articles, look for TFOS DEWS II citations in this notation: [report #, paragraph #, page #] Example: [IV, 2.3, p. 372] |
A major change in the new definition compared to the 2007 version is the addition of “a loss of homeostasis” as a disease characterization. [I, 4.3, p. 278] In the report, the subcommittee highlights a loss of tear film homeostasis as “the unifying characteristic that describes the fundamental process in the development of DED.” [I, 5.4, p. 278] According to Definition and Classification subcommittee co-chair Kelly K. Nichols, OD, MPH, PhD, the term “loss of homeostasis” was a critical element added to the definition “to reflect our current knowledge of the importance of maintaining all aspects of tear film and ocular surface equilibrium. The terminology also keeps the door open to include any elements that are found to impact the delicate tear film homeostasis as we learn more in the future about dry eye etiology.”
Another notable change is the generalization of ocular symptoms. While the 2007 DED definition specifically mentions discomfort and visual disturbance, the new definition remains more general, simply labeling DED as “accompanied by ocular symptoms.” [I, 3, p. 277; I, 4.3, p.278] This includes discomfort and visual disturbances while also accommodating the differences in symptoms reported across the world, said Dr. Craig.
In the 2007 definition, dry eye sequelae were described in terms of symptoms and tear film instability. [I, 3, p. 277] Increased tear film osmolarity and inflammation were also mentioned as factors that accompany DED. [I, 3, p. 277] However, the etiology of DED was not mentioned. [I, 3, p. 277] “It was still difficult to differentiate dry eye from other ocular diseases,” Dr. Craig said. As a result, the subcommittee added “etiological factors that are important uniquely in dry eye” and made sure to “mention those as etiological factors and not […] as diagnostic criteria,” Dr. Craig explained.
Despite several changes, the new definition still labels DED as a “multifactorial disease.” [I, 4.3, p. 278] This first appeared in the 2007 definition and remains salient in recognizing “the multitude of factors that can be involved in dry eye,” Dr. Craig said. [I, 3, p. 277]
A New Approach to Classification
The DED classification system also received a reboot to address some prevailing issues (Figure 1). The structure of the previous system led many to believe that the aqueous deficient and evaporative categories could not overlap. [I, 6.2, p. 279] “Clinicians recognize that aqueous deficient and evaporative dry eye can occur together, often referred to as mixed DED, and the previous 2007 classification scheme did not make this clear. Importantly, the new classification allows for a patient to have components of both, on a sliding scale,” says Dr. Nichols. “This now may require management of both in any one patient.”
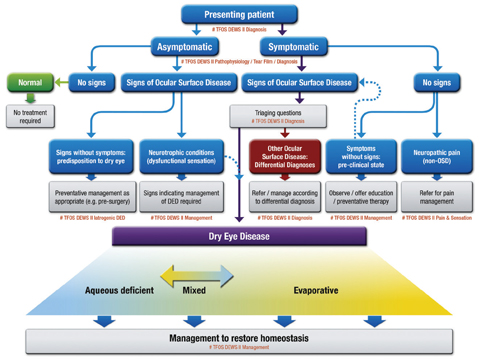 |
Fig. 1. CLASSIFICATION OF DRY EYE DISEASE. The upper portion represents a clinical decision algorithm, beginning with the assessment of symptoms and followed by review for signs of ocular surface disease. DED exhibits both symptoms and signs, and can be differentiated from other ocular surface disease with the use of triaging questions and ancillary testing. It is to this DED group that diagnostic subtyping, and conventional dry eye management strategies, apply. The lower portion of the figure represents the etiological classification of DED and highlights the two predominant and non-mutually exclusive categories: aqueous deficient dry eye (ADDE) and evaporative dry eye (EDE). Click image to enlarge. Adapted and reprinted from Ocular Surface (2017) 276–283, Craig JP, Nichols KK, Nichols JJ, et al. TFOS DEWS II definition and classification report, p. 281, © 2017, with permission from Elsevier. |
Also new to the classification system is accommodation for patients with discrepancies between signs and symptoms. The main path of the classification system includes patients who have both symptoms and signs. [I, 7.1, p. 280] This leads ODs to ask “triaging questions,” which help in diagnosing other ocular surface conditions that may require management separate from DED. [I, 7.2, p. 280]
Not all dry eye patients present with both symptoms and signs, however, and the new classification takes this into consideration. Another path represents patients with symptoms but no signs, a possibility for those presenting with “a preclinical state in which the signs are too subtle to pick up,” Dr. Craig said. [I, 7.3, p. 280; I, 7.4, p. 281] This situation could also come about if there is evidence of neuropathic pain. A path for patients with signs but no symptoms represents those “who may be picked up incidentally before ocular surgery, for instance, or it may be patients that have abnormal corneal sensation,” said Dr. Craig. [I, 7.5, p. 281; I, 7.6, p. 281–282]
The final path is for patients with no symptoms and no signs. This leads to a “normal” diagnosis that requires no treatment, another new addition to the system. [I, Fig. 3, p. 281]
“Classification schemes should aid in appropriate diagnosis and subdiagnosis, and therefore targeted management,” Dr. Nichols says. “This new patient-centric approach to DED classification allows for a decision-tree to be used to guide the clinician in patient care of the DED patient.”
Epidemiology
Ten years after the initial TFOS DEWS report, a vast swath of new literature exists to help practitioners better understand the epidemiology of DED. During this second review of the literature, the TFOS DEWS II Epidemiology subcommittee summarized the studies published in the past decade and performed meta-analyses to reveal disease prevalence stratified by sex and age. [III, abstract, p. 334; III, 1, p. 335–336] Additionally, the subcommittee summarized the available evidence on disease risk factors, natural history and morbidity. [III, 1, p. 335–336]
Key points of the Epidemiology report are as follows:
• DED definitions—and, thus, estimates—vary. The subcommittee evaluated the epidemiology of dry eye based on diagnostic criteria that include symptoms, signs or both, and on a prior diagnosis of DED made by an eye care practitioner, according to the report. [III, 7, p. 363] However, “there is considerable variation in terms of how the disease has been ascertained across these studies, and we broadly grouped the studies that were available into different diagnostic groups, including diagnostic criteria used in each of the studies of those with symptomatic disease, those that looked at signs only, those that looked at both symptoms and signs, and those looking at MGD,” said Fiona Stapleton, MCOptom, PhD, lead author of the report, during the ARVO special session. She explained that the wide variation in symptomatic disease vs. signs-only disease that exists in the literature over the past decade—with more studies focusing on signs of DED than symptoms—complicated their task of assessing disease prevalence.
Perhaps, said Dr. Stapleton in a follow-up interview, the new TFOS DEWS II dry eye definition will help to inform future epidemiological studies. “The definition of a disease needs to be ‘operationalized’ or made specific for use in epidemiological studies,” explains Dr. Stapleton, “so that in using a working diagnosis (e.g., Schirmer <5mm, corneal staining above a grade of 1, OSDI symptom score above 22), cases can be separated from non-cases.”
“Further,” adds Dr. Nichols, “our understanding of the factors that play a key role in the etiology—for example, those found in the DED definition—are largely explored through studies based in epidemiology.”
• Regardless of definition, influencing factors remain constant. The subcommittee found sex, age and geographic location remain key factors for prevalence, regardless of diagnostic criteria used. [III, 4.2, p. 348–356] Prevalence increases linearly with age, for example, and females are more frequently affected, according to the report. [III, 3.2, p. 347; III, 4.2, p. 350] Prevalence appears higher in Asian than in Caucasian populations, though studies have not been conducted in all major geographic regions, the report says. [III, 4.2, p. 341; III, 4.4, p. 352]
Other factors that affect DED prevalence include diabetes and other systemic diseases, contact lens wear, environmental exposures, computer screen use and refractive surgery, the report says. [III, 4.2, p. 348–356]
In addition, “a meta-analysis of the published data to determine prevalence of dry eye stratified by age and sex shows a rate of change per decade between 8% and 10%; so, a high rate of change in symptomatic disease” was found, Dr. Stapleton said at ARVO.
• Sex matters, except in meibomian gland dysfunction (MGD). Looking at signs-based MGD stratified by sex and age, the report shows no female predilection in this group exists, explained Dr. Stapleton. In fact, “a different trend was observed for MGD than for other dry eye diagnostic criteria; males had a slightly higher prevalence for most age categories, although the differences were not statistically significant, except in the age 80+ group,” according to the report. [III, 3.2, p. 347] However, the subcommittee notes only two studies reported age and sex data for MGD, possibly skewing the findings. “The effects of sex become more significant with age, and there does not appear to be the female preponderance in MGD as there is with other working diagnoses of dry eye disease, although this does require confirmation,” Dr. Stapleton said at ARVO.
• Kids and adults under 40 might be at risk for DED. “Most studies—again, using signs-based disease—showed an increased prevalence with age; however, symptoms were certainly high in the studies that looked at younger age groups,” said Dr. Stapleton at ARVO. Limited studies have been carried out in youth, and there remains a need for studies in populations under 40 years of age, according to the report. [III, 7, p. 363] “The high symptom reporting in youths described by clinicians requires further exploration,” Dr. Stapleton mentioned in her talk.
Elaborating, Dr. Stapleton says, “there were two studies, both in Southeast Asia, which suggested high rates of symptomatic DED in children and young adults. The vast majority of studies have included only individuals older than 40, so the rate of dry eye in youth represents a significant unanswered question and the impact of digital device use in this population requires further study.”
• Geographical mapping reveals regional variations. The subcommittee looked at prevalence data from a novel geographical perspective. This approach will facilitate future exploration of climate, socioeconomic and environmental factors. [III, 3.2, p. 342, 347]
Despite these advances, many epidemiological questions remain unanswered. While consistent risk factors were confirmed, more hypothesis-driven studies are needed to evaluate digital device use, genetics and environment. [III, 5.1, 5.2, p. 357; III, 7, p. 363] In addition, little in the way of incidence studies exist, and more populations need to be studied. “The natural history is still unknown, and this is a key area to understand the disease prognosis,” concludes Dr. Stapleton.
Iatrogenic dry eye: when a treatment becomes a triggerThe Iatrogenic subcommittee defines this form as “dry eye induced unintentionally by medical treatment from a physician or a health-related professional,” Subcommittee Chair José Alvaro Gomes, MD, said during the ARVO session. [VII, 1, p. 516–517] To gain a better understanding of contemporary iatrogenic causes, the subcommittee developed an iatrogenic dry eye classification system of the following categories: ophthalmic surgery, pharmaceuticals, contact lenses (CLs), non-surgical ophthalmic procedures and non-ophthalmic conditions. [VII, 3, p. 517]
Findings in ophthalmic surgery-induced dry eye are among the most notable of the report. The authors took a comprehensive look at literature on refractive surgery, keratoprosthesis, cataract surgery, lid surgery and more. [VII, 4.4.1, p. 524; VII, 4.4.2, p. 525; VII, 4.4.3, p. 525–526; VII, 4.4.4, p. 526–528] Post-refractive surgery links to dry eye include the effect of a neurotrophic component on the lacrimal functional unit, as well as ocular rosacea’s tendency to reduce tear break-up time. [VII, 4.4.1, p. 524] In keratoprosthesis, post-procedure reorganization of the nerves surfaced as a possible culprit, Dr. Gomes said. Literature on cataract surgery showed topical anesthetics and desiccation, possible light toxicity from the operating microscope, nerve transection, elevation of inflammatory factors, goblet cell loss and MGD as potential dry eye contributors.[VII, 4.4.2, p. 525] Also, a close interaction of the eyelid, tear film and ocular surface showed some prevalence in lid surgery-related dry eye. [VII, 4.4.3, p. 525–526] Drug-induced dry eye can be related to either topical or systemic medications. [VII, 3, p. 517] For topical drugs (Table 1), the subcommittee found that the concentration of preservatives in glaucoma medications, specifically benzalkonium chloride, has the potential to cause inflammation and proptosis. [VII, 4.2.3, p. 521] “We know also that these patients sometimes don’t use one—they use two or three medications, and this causes a potential effect of toxicity and dry eye,” Dr. Gomes said. Of the top 100 best-selling systemic drugs in the United States in 2009, 22 proved to possibly cause dry eye secondary to decreased tear production, altered nerve input and reflex secretion, inflammatory effects on secretory glands or direct irritation effects through secretion into the tears, according to the report. [VII, 4.1.1, p. 517] Factors involved in CL-induced dry eye include biophysical changes to the tear film such as a thinner lipid layer and increased tear evaporation, and ocular responses such as alterations to Langerhans cells, conjunctival goblet cell density and lid wiper epitheliopathy. [VII, 4.3.2, p. 524] Recall that existing DED can be exacerbated by lens wear and lenses can induce a dry eye state. Paul Karpecki, OD, who co-chaired the TFOS contact lens session and served on the TFOS DEWS II Diagnostic Methodology committee, noted studies by Villani and Arita showing that contact lenses can cause structural changes to the meibomian glands but said “it is up to the clinician to monitor for these and intervene before functional changes ensue.” Environmental circumstances such as computer use in an office setting also seemed to be a contributing factor to dry eye. [VII, 4.3.2, p. 524] In each instance, careful exploration of symptoms and signs are warranted to detect early stages of DED, Dr. Karpecki noted. Some nonsurgical ophthalmic procedures that show dry eye prevalence include botulinum toxin treatment, corneal collagen crosslinking, positive pressure noninvasive ventilation, radiation treatment and cosmetic procedures such as eye makeup, tattooing and piercing. [VII, 4.5.1, p. 528; VII, 4.5.2, p. 529; VII, 4.5.3, p. 529; VII, 4.5.4.1, p. 530; VII, 4.5.4.2, p. 530] |

